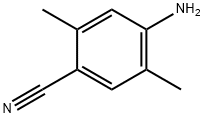
2,5-DiMethyl-4-aMinobenzonitrile synthesis
- Product Name:2,5-DiMethyl-4-aMinobenzonitrile
- CAS Number:72917-37-4
- Molecular formula:C9H10N2
- Molecular Weight:146.19
Yield:72917-37-4 52%
Reaction Conditions:
with tetrakis-(triphenylphosphine)-palladium in N,N-dimethyl-formamide at 120; for 48 h;Sealed tube;
Steps:
6B Example 6B: Preparation of 4-amino-2,5-dimethylbenzonitrile.
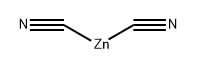
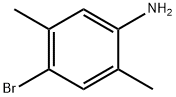
A solution of 4-bromo-2,5-dimethylaniline (15 g, 75.00 mmol) and Zn(CN)2 (9.6 g, 82.50 mmol) in DMF (150 mL) was degassed for 10 min. Tetrakis(triphenylphosphine)- palladium(O) (12.9 g, 11.25 mmol) was then added, and the reaction mixture was heated to 120 °C for 2 days in a sealed tube. After 2 d, the reaction mixture was poured into ice cold water (400 mL) and extracted with EtOAc (3 x 600 mL). The combined organic layers were dried over anhydrous Na2SC>4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel (S1O2), 15 ->20% ethyl acetate in petroleum ether) to afford the title compound (5.7 g, 52% yield) as pale yellow solid: NMR (400 MHz, CDCb) d 7.25 (s, 1H), 6.50 (s, 1H), 3.98 (brs, 2H), 2.40 (s, 3H), 2.11 (s, 3H); ESIMS m/z 147 ([M+H]+).
References:
WO2020/237131,2020,A1 Location in patent:Paragraph 0085
73713-69-6
21 suppliers
inquiry

72917-37-4
23 suppliers
inquiry
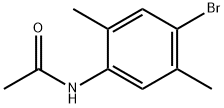
13711-31-4
24 suppliers
$28.60/50MG
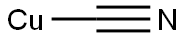
544-92-3
246 suppliers
$9.00/1g

72917-37-4
23 suppliers
inquiry
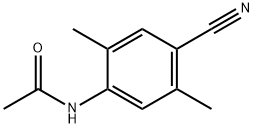
94881-68-2
0 suppliers
inquiry