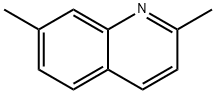
2,5-Dimethylquinoline synthesis
- Product Name:2,5-Dimethylquinoline
- CAS Number:26190-82-9
- Molecular formula:C11H11N
- Molecular Weight:157.21

64-17-5
706 suppliers
$10.00/50g
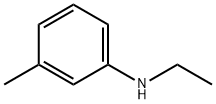
108-44-1
365 suppliers
$9.00/1g
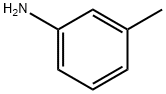
93-37-8
64 suppliers
$295.00/1g

26190-82-9
24 suppliers
inquiry
Yield:93-37-8 51% ,26190-82-9 45%
Reaction Conditions:
Stage #1: ethanolwith iron chromite;dihydrogen peroxide in water at 20; for 2 h;UV-irradiation;
Stage #2: 1-amino-3-methylbenzene in water; for 0.0833333 h;
Steps:
Photoactivated tandem synthesis of substitutedquinolines.
General procedure: Synthesis with the use of photoactivation was carried out in a photocatalytic installation Photo Catalytic Reactor Lelesil Innovative Systems with a quartz reactor of 500 mL capacity (Strohmeier type photoreactor with a magnetic stirrer). The reactor was charged with 4.8 mmol of catalyst Fe(CrO2)2 and 120 mmol of an appropriate alcohol, the reactor was shaken and left standing for 24 h to reach an equilibrium. Just before the reaction the dispersion of catalyst in the alcohol was subjected to microwave irradiation for 10 min(Ultrasound bath “Sapfir”, power 55 W). 102 mL of 5% aqueous solution of H2O2 (150 mmol of hydrogen peroxide) was added to the treated dispersion. The reactor was attached to the installation in keeping with the instructions of the producer. Light source was a Hg-lamp of moderate pressure of the power 250 W. Spectral composition of radiation by energy was as follows: 48% UV range, 43% visible range, and 9% IR range. Spectral range 222-1368 nm. The light beam went to the system through a temperature-controlled water layer (20°C). Photoactivation time 120 min. Then to the reaction mixture 50 mmol of arylamine was added, the mixture was stirred for 5 min and worked up as in the experiments without photoactivation.
References:
Makhmutov [Russian Journal of Organic Chemistry,2018,vol. 54,# 8,p. 1166 - 1172][Zh. Org. Khim.,2018,vol. 54,# 8,p. 1156 - 1161,6]

108-44-1
365 suppliers
$9.00/1g

123-73-9
198 suppliers
$40.00/5g

93-37-8
64 suppliers
$295.00/1g

26190-82-9
24 suppliers
inquiry

75-07-0
417 suppliers
$14.00/5mL

108-44-1
365 suppliers
$9.00/1g

93-37-8
64 suppliers
$295.00/1g

26190-82-9
24 suppliers
inquiry

123-73-9
198 suppliers
$40.00/5g

108-44-1
365 suppliers
$9.00/1g

93-37-8
64 suppliers
$295.00/1g

26190-82-9
24 suppliers
inquiry