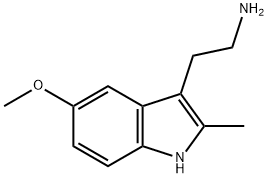
2-(5-Methoxy-2-Methyl-1H-indol-3-yl)ethanaMine synthesis
- Product Name:2-(5-Methoxy-2-Methyl-1H-indol-3-yl)ethanaMine
- CAS Number:3143-97-3
- Molecular formula:C12H16N2O
- Molecular Weight:204.27
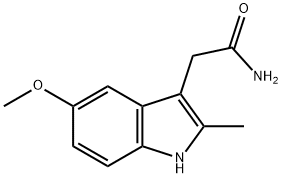
15992-10-6
31 suppliers
$17.00/100mg

3143-97-3
7 suppliers
inquiry
Yield:3143-97-3 55%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 144 h;
Steps:
7
[0258] 2-(5-Methoxy-2-methyl-1H-indol-3-yl)-ethylamine (Compound 268). Compound 303 (273 mg, 0.7 mmol) was dissolved in freshly distilled THF (30 ml) and cooled to 0° C. Slow addition of a 1 M solution of LAH (0.85 ml, 0.85 mmol) was made with vigorous gaseous evolution noted. The reaction was stirred at room temperature (RT) for 6 days (144 hours), after which time it was poured slowly onto ice water and diluted with ether (150 ml). The aqueous layer was washed with ether (2×150 mL) and all ether extracts were combined and acidified with HCl (1 N, 3×150 mL). The acid extracts were treated with 4 N NaOH until pH 10 and the products were extracted into ether (3×150 mL), dried, and concentrated to give selectively 1-(4-bromobenzyl)-5-methoxy-2-methyl-3-indolethylamine in 55% yield. No 1-benzyl-5-methoxy-2-methyl-3-indolethylamine was detected.
References:
US2005/2859,2005,A1 Location in patent:Page/Page column 22
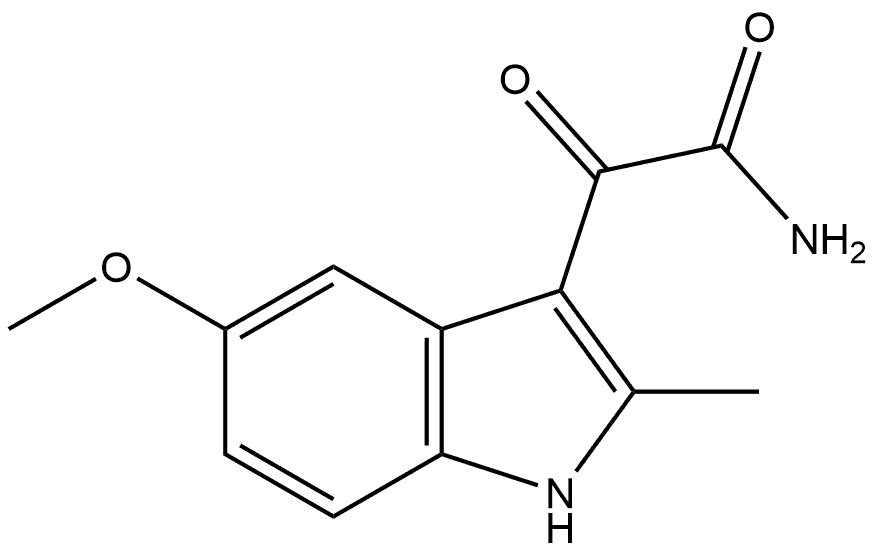
124224-49-3
0 suppliers
inquiry

3143-97-3
7 suppliers
inquiry
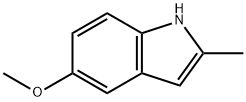
1076-74-0
200 suppliers
$15.00/1g

3143-97-3
7 suppliers
inquiry
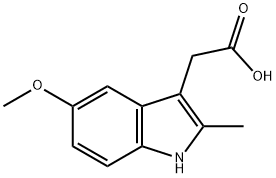
2882-15-7
155 suppliers
$12.00/100mg

3143-97-3
7 suppliers
inquiry
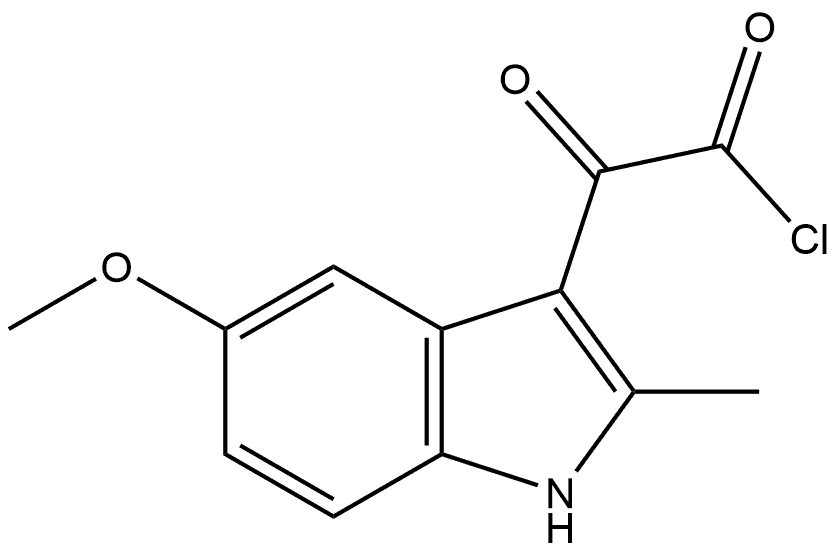
1026388-93-1
0 suppliers
inquiry

3143-97-3
7 suppliers
inquiry