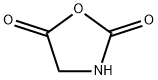
2,5-OXAZOLIDINEDIONE synthesis
- Product Name:2,5-OXAZOLIDINEDIONE
- CAS Number:2185-00-4
- Molecular formula:C3H3NO3
- Molecular Weight:101.06
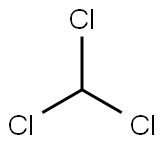
67-66-3
6 suppliers
$33.60/500ml
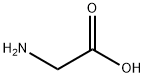
56-40-6
1211 suppliers
$5.00/25g

2185-00-4
93 suppliers
$86.00/1 / G
Yield: 56%
Reaction Conditions:
with oxygen in acetonitrile at 60;UV-irradiation;
Steps:
15 Example 15: Synthesis of glycine-N-carboxylic acid anhydride
Purified chloroform (15 mL, 186 mmol) in the reaction vessel of the reaction system used in Example 1,Acetonitrile (15 mL, 287 mmol),And glycine (375 mg, 5.0 mmol),Mix with stirring.While stirring the reaction solution,Oxygen gas at 0.5 L/min was bubbled at 60°C by bubbling. Under stirring conditions of the solution,Irradiate high-energy light including UV-C from a low-pressure mercury lamp for 5 minutes,Subsequently, the cycle of stopping the irradiation for 10 minutes was repeated 12 times in total to carry out the reaction. Then, dichloromethane (320 μL, 5.0 mmol) was added to the reaction solution as an internal standard, and the result was analyzed by 1 H NMR. As a result, formation of the target compound, glycine-N-carboxylic acid anhydride, could be confirmed.(Yield: 56%).
References:
National University Corporation Kobe University;Akihiko, Tsuda JP2020/83882, 2020, A Location in patent:Paragraph 0080