
2-(6-Chloropyridin-2-yl)ethanaMine synthesis
- Product Name:2-(6-Chloropyridin-2-yl)ethanaMine
- CAS Number:933734-79-3
- Molecular formula:C7H9ClN2
- Molecular Weight:156.61
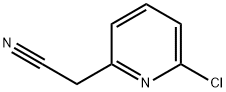
75279-60-6
48 suppliers
inquiry

933734-79-3
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran at 0 - 20; for 21 h;Reflux;
Steps:
20
To a cold solution of Intermediate BZ (1.0 mmol) in THF (30 mL) at 0 °C was BH3?DMS (9.0 mmol) was added slowly over 5 minutes. After the addition was complete, the reaction mixture was allowed to reach room temperature and stirred for 20 hours. The reaction mixture was cooled in ice and then quenched with MeOH (5 mL). The reaction was then refluxed for 1 hour and concentrated. The residue was dissolved in ethyl acetate (50 mL), washed with water (2 x 10 mL) and brine solution (15 mL), dried over anhydrous Na2S04, and the solvent was removed to afford the crude Intermediate CA (Table 20), which was in the next step without further purification.
References:
WO2011/47156,2011,A1 Location in patent:Page/Page column 98-99
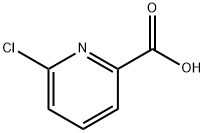
4684-94-0
326 suppliers
$5.00/1g

933734-79-3
7 suppliers
inquiry
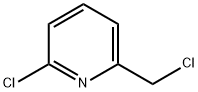
78846-88-5
72 suppliers
$65.00/50mg

933734-79-3
7 suppliers
inquiry
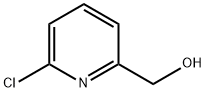
33674-97-4
130 suppliers
$8.00/1g

933734-79-3
7 suppliers
inquiry