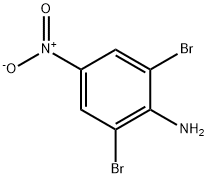
2,6-Dibromo-4-nitroaniline synthesis
- Product Name:2,6-Dibromo-4-nitroaniline
- CAS Number:827-94-1
- Molecular formula:C6H4Br2N2O2
- Molecular Weight:295.92

100-01-6
10 suppliers
$11.19/25G

827-94-1
229 suppliers
$8.00/25g
Yield:827-94-1 98.7%
Reaction Conditions:
with hydrogenchloride;brominating reagent in dichloromethane;water at 28; for 0.75 - 1 h;
Steps:
5 EXAMPLE 5
To 5.0 ml of dichloromethane containing 4-nitroaniline (1 g, 7.246 m mole) in a 250 ml round bottom flask, 1.45 ml of 12 N hydrochloric acid and 10 ml of deionised water were added. To this reaction mixture, brominating reagent containing (2.9 g) of brominating reagent dissolved in 20 ml of deionised water was added slowly under continuous stirring at 28° C. for a period of 30 to 45 minutes. After completion of the addition, stirring was continued for another 15 minutes. The organic layer was separated and extracted with dichloromethane. The organic layer and the organic extracts were mixed and then washed successively with sodium thiosulphate solution and brine. The product, 2,6-dibromo-4-nitroaniline was dried over anhydrous sodium sulphate and concentrated to yield 98.7%. It was characterized by melting point; NMR; IR and elemental analysis. _
References:
COUNCIL OF SCIENTIFIC AND INDUSTRIAL RESEARCH US2004/242939, 2004, A1 Location in patent:Page 4
13296-94-1
175 suppliers
$6.00/1g

827-94-1
229 suppliers
$8.00/25g

96-75-3
157 suppliers
$13.00/25g

827-94-1
229 suppliers
$8.00/25g

31106-74-8
27 suppliers
$131.00/1g

827-94-1
229 suppliers
$8.00/25g