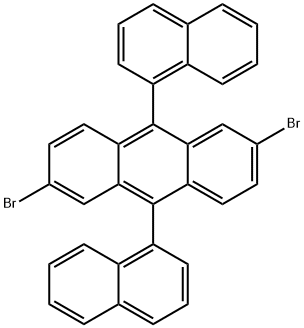
2,6-Dibromo-9,10-di(naphthalen-1-yl)anthracene synthesis
- Product Name:2,6-Dibromo-9,10-di(naphthalen-1-yl)anthracene
- CAS Number:914306-89-1
- Molecular formula:C34H20Br2
- Molecular Weight:588.33
Yield:914306-89-1 83.1%
Reaction Conditions:
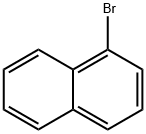
Stage #1: 1-Bromonaphthalene;2,6-dibromoanthraquinonewith magnesium in tetrahydrofuran; for 6 h;Reflux;
Stage #2: with tin(ll) chloride in N,N-dimethyl-formamide; for 5 h;Reflux;
Steps:
6.a a) 2,6-Dibromo-9, 1 0-bis(naphth- 1 -yl)anthracene
a)
2,6-Dibromo-9,10-bis(naphth-1-yl)anthracene
The corresponding Grignard reagent is prepared from 30.5 ml (220 mmol) of 1-bromonaphthalene and 5.5 g (225 mmol) of magnesium in 500 ml of THF. 36.6 g (100 mmol) of 2,6-dibromoanthraquinone are added to this Grignard reagent, the mixture is refluxed for 6 h and allowed to cool, 15 ml of acetic acid are added, the mixture is evaporated to dryness, the residue is taken up in 500 ml of DMF, 56.9 g (300 mmol) of tin(II) chloride are added, and the mixture is refluxed for 5 h.
After cooling, 200 ml of 2N hydrochloric acid are added, the mixture is stirred for a further 1 h, the solid is filtered off with suction, washed three times with 200 ml of 2N hydrochloric acid each time, three times with 300 ml of water each time, and three times with 200 ml of ethanol each time, dried under reduced pressure and recrystallised once from DMF. Yield: 48.9 g (83 mmol), 83.1% of theory; purity: 98% according to 1H-NMR.
References:
US2015/322091,2015,A1 Location in patent:Paragraph 0190; 0191
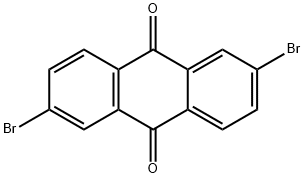
633-70-5
200 suppliers
$7.00/250mg

914306-89-1
28 suppliers
inquiry
1163732-62-4
1 suppliers
inquiry

914306-89-1
28 suppliers
inquiry