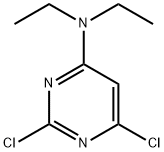
2,6-DICHLORO-N,N-DIETHYLPYRIMIDIN-4-AMINE synthesis
- Product Name:2,6-DICHLORO-N,N-DIETHYLPYRIMIDIN-4-AMINE
- CAS Number:78418-15-2
- Molecular formula:C8H11Cl2N3
- Molecular Weight:220.1
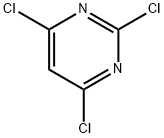
3764-01-0
402 suppliers
$6.00/5g

109-89-7
451 suppliers
$10.00/5g

7038-63-3
19 suppliers
inquiry

78418-15-2
24 suppliers
inquiry
Yield:78418-15-2 62%
Reaction Conditions:
with triethylamine in ethanol at 0; for 0.5 h;
Steps:
1.1
Example 1. Synthesis of Compound 30. The synthesis of the 30 consists of 4 steps. The following scheme depicts the synthetic route: EPO
References:
WO2006/53109,2006,A1 Location in patent:Page/Page column 99-100

10132-07-7
300 suppliers
$10.00/1g

75-03-6
355 suppliers
$11.00/5g

78418-15-2
24 suppliers
inquiry

70958-39-3
19 suppliers
inquiry