
2,6-DifluorophenylMethanol-d2 synthesis
- Product Name:2,6-DifluorophenylMethanol-d2
- CAS Number:1346601-28-2
- Molecular formula:C7H6F2O
- Molecular Weight:144.1187
13671-00-6
167 suppliers
$9.00/5g

1346601-28-2
6 suppliers
$165.00/25mg
Yield:1346601-28-2 1.77 g
Reaction Conditions:
with deutero lithium aluminiumhydride in tetrahydrofuran at 0; for 0.75 h;
Steps:
162A (2,6-Difluorophenyl)(2H2)methanol
Example 162A
(2,6-Difluorophenyl)(2H2)methanol
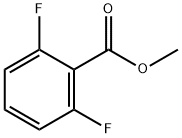
2.0 g of methyl 2,6-difluorobenzoate (11.6 mmol, 1 equivalent) were initially charged in 40 ml of THF at 0° C., and at this temperature 23.2 ml of 1 M lithium aluminium deuteride solution in THF (23.2 mmol, 2 equivalents) were added slowly.
The mixture was stirred for 45 min, and 1.2 ml of water, 1.2 ml of 2 N aqueous sodium hydroxide solution and 2.3 ml of water were then added in succession.
The resulting precipitate was filtered off and washed well with THF.
The filtrate was concentrated and the residue was reacted further without purification.
This gave 1.77 g (104% of theory) of the title compound.
GC-MS (Method 6): Rt=2.38 min
MS (EIpos): m/z=146.1 (M)+
1H NMR (400 MHz, DMSO-d6): δ=5.20 (s, 1H), 7.02-7.12 (m, 2H), 7.24-7.44 (m, 1H).
References:
US2014/128372,2014,A1 Location in patent:Paragraph 0853; 0854; 0855; 0856; 0857