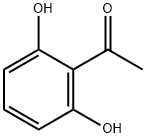
2',6'-Dihydroxyacetophenone synthesis
- Product Name:2',6'-Dihydroxyacetophenone
- CAS Number:699-83-2
- Molecular formula:C8H8O3
- Molecular Weight:152.15

108-58-7
138 suppliers
$14.00/1g

699-83-2
467 suppliers
$6.00/5g
Yield:699-83-2 60%
Reaction Conditions:
with hydrogen fluoride supported on silica gel in neat (no solvent) at 55; for 4 h;Green chemistry;
Steps:
3.4. General Procedure for Synthesis of Hydroxyaryl Ketones
General procedure: A mixture of aryl ester (25 mmol), HFSiO2 (2 g), and EtOAc (5 mL) was charged in a 50 ml flask and stirred for 5min. EtOAc was used for homogenization of the reactionmixture in this step. The solvent was evaporated under vacuumand the residue was heated with the classical method for 4 h at 55°C for aryl acetates and at 75°C for aryl benzoates(Table 1). After cooling, the reaction mixture was extractedwith EtOAc (3 × 10 mL) and the solvent was removed under vacuum. The residue was subjected to short column chromatography (EtOAc/hexane; 1:5) on silica gel to obtain pureproducts. All the isolated hydroxyaryl ketones successfullygave the related spectral data of IR, 1H NMR, 13C NMR andMS spectrometers (see the Supporting Information, Fig. 12S-39S) and comparison with authentic samples prepared byreported methods [33-36].
References:
Paghandeh, Hossein;Saeidian, Hamid;Ghaffarzadeh, Mohammad [Letters in Organic Chemistry,2018,vol. 15,# 9,p. 809 - 814]

75-36-5
569 suppliers
$17.92/100G
95-50-1
580 suppliers
$10.00/5g
108-46-3
755 suppliers
$10.00/10g

699-83-2
467 suppliers
$6.00/5g

108-46-3
755 suppliers
$10.00/10g

699-83-2
467 suppliers
$6.00/5g
![Ethanone, 1-[2,6-bis(acetyloxy)phenyl]-](/CAS/20180601/GIF/144152-28-3.gif)
144152-28-3
0 suppliers
inquiry

699-83-2
467 suppliers
$6.00/5g

2555-29-5
87 suppliers
$130.00/500mg

699-83-2
467 suppliers
$6.00/5g