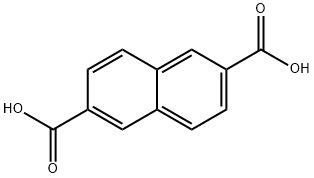
2,6-NAPHTHALENEDICARBOXYLIC ACID synthesis
- Product Name:2,6-NAPHTHALENEDICARBOXYLIC ACID
- CAS Number:1141-38-4
- Molecular formula:C12H8O4
- Molecular Weight:216.19
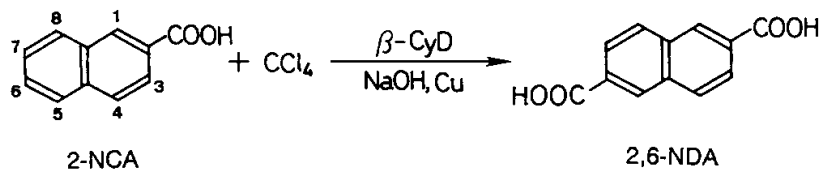
Three mmol of 2-naphthalenecarboxylic acid/2-NCA), 0,8 mmol(0,05 g) of copper powder, and 3,0 mmol of β-CyD were added to 30 mL of 30 wt.-% aqueous sodium hydroxide solution. The reaction was started with the addition of 8,5 mmol of carbon tetrachloride and was continued at 60°C for 7 h with magnetic stirring under nitrogen. Then, the residual carbon tetrachloride was removed by evaporation under reduced pressure.
581-42-0
169 suppliers
$50.00/1 g

1141-38-4
250 suppliers
$5.00/250mg
Yield:1141-38-4 95.46%
Reaction Conditions:
with oxygen;manganese;bromine;cobalt in water;acetic acid at 200; under 14711.4 Torr;Product distribution / selectivity;
Steps:
1
A titanium reactor having a capacity of 300 L, which is equipped with a cooler, a heater and a stirrer, was charged with reactants and catalyst, and an oxidation reaction was performed. The oxidation reactor was set to a temperature of 200°C and a pressure of 20 kg/cm2, and the stirrer was rotated at 700 rpm to disperse the reactant gases. During the reaction, air was introduced in an amount of about 35.7 moles per mole of 2,6-dimethylnaphthalene until the initial amount of the gas being recycled was secured, and was introduced in an amount of 22 moles per mole of 2,6-dimethylnaphthalene after the system was stabilized. As a diluent gas, nitrogen was introduced in an amount of 1.8 moles per mole of 2,6-dimethylnaphthalene, and after stabilization, the amount of the discharged gas to be recycled was adjusted such that the oxygen content in the discharged gas was 4 to 6% by weight. After the steps of oxidation reaction followed by crystallization and solid-liquid separation, the final product was obtained, and the final product was purged with BSTFA in a nitrogen environment and then subjected to analysis by gas chromatography. The amount of the mother liquor recycled during the production was adjusted to 10% by weight. The amount of 2,6-dimethylnaphthalene used, the amount of acetic acid and the amount of catalyst are presented in Table 1, while the yield of the final product and the content of impurities are presented in Table 2.; Table 1 Example 1 Example 2 Example 3 Comp. Ex. 1 Comp. Ex. 2 2,6-Dimethylnaphthalene 5 kg/hr 5 kg/hr 5 kg/hr 5 kg/hr 5 kg/hr Acetic acid 88 kg/hr 84 kg/hr 79 kg/hr 69 kg/hr 87 kg/hr Cobalt 6.21 wt% 5.0 wt% 5.0 wt% 5.0 wt% 5.0 wt% Manganese 1.58 wt% 1.0 wt% 1.0 wt% 1.0 wt% 1.0 wt% Bromine 3.87 wt% 3.87 wt% 3.87 wt% 3.87 wt% 3.87 wt% Distilled water 25.0 wt% 23.0 wt% 23.0 wt% 23.0 wt% 23.0 wt% Amount of mother liquor recycled 10 kg/hr 5 kg/hr 10 kg/hr 20 kg/hr 2 kg/hr* The weight ratio given in Table 1 is a weight ratio relative to 100 wt% of dimethylnaphthalene. * The weight of cobalt, manganese and bromine in Table 1 is a weight of each atom, each atom is used as above mentioned the compounds, and each compound is added in accordance with the weight ratio of each atom.Table 2 Example 1 Example 2 Example 3 Comp. Ex. 1 Comp. Ex. 2 NDA 99.41 wt% 99.39 wt% 99.35 wt% 99.02 wt% 99.26 wt% TMLA 19 ppm 20 ppm 23 ppm 32 ppm 27 ppm FNA, NA, MNA 40 ppm 38 ppm 41 ppm 46 ppm 43 ppm Yield 95.46% 95.73% 96.18% 94.97% 95.01%* The yield given in Table 2 is a ratio comparing NDA with DMN (NDA/DMN) in molar units. * NDA: naphthalenedicarboxylic acid; TMLA: trimellitic acid; FNA: 2,6-formylnaphthoic acid; NA: naphthoic acid; MNA: methylnaphthalenecarboxylic acid; and DMN: 2,6-dimethylnaphthalene
References:
Hyosung Corporation EP1860092, 2007, A1 Location in patent:Page/Page column 5-6
69750-34-1
10 suppliers
inquiry

1141-38-4
250 suppliers
$5.00/250mg
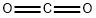
124-38-9
131 suppliers
$175.00/23402
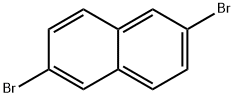
13720-06-4
233 suppliers
$41.00/1g

1141-38-4
250 suppliers
$5.00/250mg

581-42-0
169 suppliers
$50.00/1 g

1141-38-4
250 suppliers
$5.00/250mg
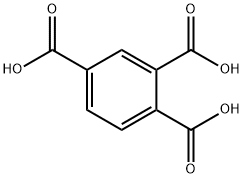
528-44-9
227 suppliers
$11.00/5g
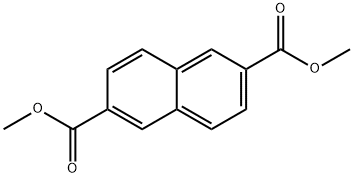
840-65-3
181 suppliers
$6.00/1g

1141-38-4
250 suppliers
$5.00/250mg