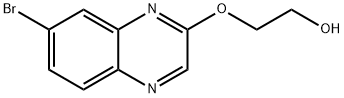
2-(7-BROMOQUINOXALIN-2-YLOXY)ETHANOL synthesis
- Product Name:2-(7-BROMOQUINOXALIN-2-YLOXY)ETHANOL
- CAS Number:705262-64-2
- Molecular formula:C10H9BrN2O2
- Molecular Weight:269.09

89891-65-6
160 suppliers
$7.00/100mg
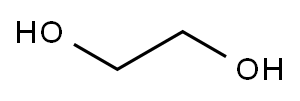
107-21-1
1316 suppliers
$10.00/25g

705262-64-2
9 suppliers
inquiry
Yield: 85%
Reaction Conditions:
Stage #1:ethylene glycol with sodium hydride in DMF (N,N-dimethyl-formamide) at 0 - 20;
Stage #2:7-bromo-2-chloroquinoxaline in DMF (N,N-dimethyl-formamide) at 20; for 2 h;
Steps:
27 Preparation 27; Preparation of 2-(7-Bromo-quinoxalin-2-yloxy)-ethanol
Preparation 27 Preparation of 2- (7-Bromo-quinoxalin-2-yloxy)-ethanol Suspend sodium hydride (310 mg, 13MMOL) in a solution of ethylene glycol (800 mg, 13 mmol) in DMF (50 mL) at 0 C. After stirring for 30 min at room temperature, 7- bromo-2-chloro-quinoxaline (Preparation 26; 610 mg, 2.5 mmol) is added. Stir for 2 h at room temperature and dilute with 3: 1 CHLOROFORM/ISOPROPYL alchol. Wash the organic phase with brine and purify by FCC to give the title compound (564 mg, 85%). MS m/e (268, M+1). LU NMR (400 MHz, CDC13) 8 8.5 (s, 1H), 8.0 (d, J = 2.0 Hz), 7.90 (dd, J = 8.8, 2. 0 HZ, 1H), 7.7 (d, J = 8. 8 HZ, 1H), 4.6 (t, D = 4. 8 HZ, 2H), 43.9 (t, J = 4. 8 HZ, 2H).
References:
ELI LILLY AND COMPANY WO2004/50659, 2004, A1 Location in patent:Page 49