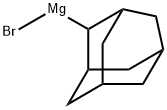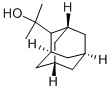
2-ADAMANTAN-2-YL-PROPAN-2-OL synthesis
- Product Name:2-ADAMANTAN-2-YL-PROPAN-2-OL
- CAS Number:105419-86-1
- Molecular formula:C13H22O
- Molecular Weight:194.31

22635-58-1
5 suppliers
inquiry

105419-86-1
1 suppliers
inquiry
Yield:105419-86-1 78%
Reaction Conditions:
with methyllithium in diethyl ether at 0 - 20;Inert atmosphere;
Steps:
2-(2-Adamantyl-2-propanol, 32.
To a stirred solution of sodium ethoxide (220 mg, 9.60mmol) in absolute ethanol (5 mL) and anhydrous THF (11 mL), a solution of 2-adamantanone (960 mg, 6.40 mmol) and toluenesulfonyl methylisocyanide (TOSMIC) (1.49 g,7.68 mmol) in anhydrous THF (25 mL) at room temperature. The mixture was stirred at room temperature for 2 h, then solvent was evaporated in vacuo andwater (15 mL) was added. The mixture was extracted with ether and the organic solution was dried (Na2SO4). Solvent was removed to afford quantitatively 2-cyanoadamantane 4 (ethanol - water). To a stirred solution of 2-cyanoadamantane 4 (350 mg, 2.17 mmol) in anhydrous ether was added during a 2 min period, under an argon atmosphere, a solution of MeLi 1.6 M in ether (4.1 mL, 6.56 mmol) at 0 °C. The yellow complex formed was stirred for 90 min at ambienttemperature and hydrolyzed with water (8 mL) at 0 C. The residue was treated with a mixture of acetone (5 mL) and HCl 6N (5 mL) and the mixture was refluxed for 2 h. Acetone was removed in vacuo and the resulting mixture was extracted with ether. The organic solution was washed with water and brine, was dried (Na2SO4) and evaporated in vacuo to afford 2-acetyladamantane 34 (302 mg, 78 %). To a stirred solution of 2-acetyladamantane 34 (590 mg, 3.31 mmol) in anhydrous ether (40 mL) was added dropwise, under an argon atmosphere, a solution of MeLi 5 % in ether (4.4 mL, 10.0 mmol) at 0 °C. The mixture was stirred overnight at ambient temperature, then a saturated solution of NH4Cl (50 mL) was added at 0 °C. The organic phase was separated, washed with water, brine and dried over Na2SO4. After solvent evaporation the liquid alcohol 32 (502 mg, 78 %).
References:
Kolocouris, Antonios;Koch, Andreas;Kleinpeter, Erich;Stylianakis, Ioannis [Tetrahedron,2015,vol. 71,# 16,p. 2463 - 2481] Location in patent:supporting information
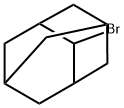
7314-85-4
161 suppliers
$18.00/1g

105419-86-1
1 suppliers
inquiry
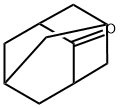
700-58-3
450 suppliers
$5.00/5g

105419-86-1
1 suppliers
inquiry

35856-00-9
15 suppliers
inquiry

105419-86-1
1 suppliers
inquiry