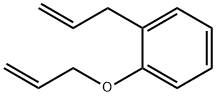
2-ALLYL PHENYL ALLYL ETHER synthesis
- Product Name:2-ALLYL PHENYL ALLYL ETHER
- CAS Number:3383-05-9
- Molecular formula:C12H14O
- Molecular Weight:174.24
Yield:3383-05-9 98%
Reaction Conditions:
with 5%-palladium/activated carbon;potassium carbonate;triphenylphosphine in water at 85 - 105; for 16 h;Inert atmosphere;
Steps:
1 Synthesis of substrate(2-allylphenol allyl ether)
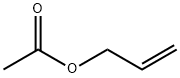
A 3 L three-necked round bottom flask was charged with a solution of 567 g (4.10 mol) of potassium carbonate (manufactured by Nippon Soda Co., Ltd.) in 750 g of pure water, a solution of 2-allylphenol (manufactured by Tokyo Chemical Industry Co., ) 500 g (3.73 mol), and the reactor was purged with nitrogen gas and heated to 85 ° C. 448 g (4.47 mol) of allyl acetate (manufactured by Showa Denko KK), 9.77 g (37.3 mmol) of triphenylphosphine (manufactured by Hokko Chemical Industry Co., Ltd.) and 50% water-containing 5% - Pd / C- 3.17 g (0.750 mmol (as Pd atom)) of STD type (manufactured by NI E Chemcat Corporation) was placed and heated to 105 ° C. in a nitrogen gas atmosphere and reacted for 4 hours, then allyl acetate 44. 8 g (0.447 mol) was added additionally, and heating was continued for 12 hours.After completion of the reaction, the reaction system was cooled to room temperature, pure water was added until all the precipitated salt was dissolved, and liquid separation treatment was carried out.The organic layer was separated and the organic solvent (70 ° C., 50 mmHg, 2 hours) was distilled off. After addition of purified water (500g), toluene 500g added, after confirming that the white precipitate is not deposited and held above 80 ° C. of temperature, filtered Pd / C (1 micron membrane filter (Advantec Pressure (0.3 MPa) using KST-142-JA manufactured by Kyowa Interface Science Co., Ltd.).The filter cake was washed with 100 g of toluene, and the aqueous layer was separated.The organic layer was washed twice with 500 g of pure water at 50 ° C. or higher, and it was confirmed that the aqueous layer was neutral.Obtained after separation of the organic layer under reduced pressure, concentrated, pale yellow liquid composed mainly of 2-allylphenol allyl ether represented by the formula (5) (669g, 3.66mol, 98.0% yield) It was.
References:
JP2018/2691,2018,A Location in patent:Paragraph 0053

51230-30-9
0 suppliers
inquiry

3383-05-9
2 suppliers
inquiry

106-95-6
425 suppliers
$10.00/5g
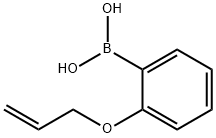
151414-76-5
21 suppliers
$435.00/250mg

3383-05-9
2 suppliers
inquiry

107-05-1
404 suppliers
$16.80/100ML

108-95-2
734 suppliers
$14.00/25g
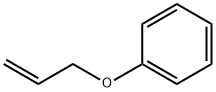
1746-13-0
233 suppliers
$9.00/5g

3383-05-9
2 suppliers
inquiry

68714-32-9
0 suppliers
inquiry
1745-81-9
267 suppliers
$14.00/5g