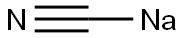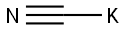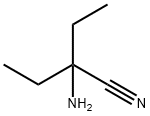
2-amino-2-ethylbutanenitrile synthesis
- Product Name:2-amino-2-ethylbutanenitrile
- CAS Number:22374-51-2
- Molecular formula:C6H12N2
- Molecular Weight:112.17
Yield:22374-51-2 89%
Reaction Conditions:
with ammonia;ammonium chloride in water at 20; for 72 h;
Steps:
1
Preparation of 3-[(1-Carbamoyl-1-ethyl)propylamino]-1-propanesulfonic acid (Compound QF); To a 250 mL I neck flask containing 30% NH4OH (120 mL) was added NaCN (15.34 g, 0.31 mol) and NH4Cl (19.75 g, 0.37 mol) with vigorous stirring. The corresponding ketone was added dropwise within 20 minutes at room temperature. The mixture was stirred for 3 days at room temperature follwed by extraction with dichloromethane (50 mL). The organic layer was separated and dried over anhydrous sodium sulfate for 2 hours. The sodium sulfate was removed by filtration, the solvent was removed under reduced pressure to yield the crude aminonitrile. The desired material was obtained as an light brown oil (colorless oil, 89% crude yield). 1H NMR (500 MHz, DMSO-d6) δ 0.95 (t, J=7.6 Hz, 6H), 1.52 (hex, J=7.2 Hz, 2H), 1.61 (hex, J=7.3 Hz, 2H), 2.39 (br s, 2H); 13C NMR (125 MHz, DMSO-d6) δ 8.3, 31.9, 54.6, 124.2 To 10 g of concentrated sulfuric acid stirred in an ice cooled water bath was added dropwise a solution of the aminonitrille (41 mmol) in 30 mL CH2Cl2, maintaining the internal temperature at 15° C. Then the bath was removed and the mixture heated to 40° C. for 1 hour. The mixture was cooled in ac ice bath and poured onto 200 g of crushed ice. The mixture was made pH 7-8 with 28% aqueous NH3 and extracted with EtOAc (3×100 mL). The extracts were collected, dried (MgSO4), and evaporated to dryness. The crude solid was recrystallized in EtOAc/Hex. The desired material was obtained as a white foamy solid 0.941 g, 7.23 mmol, 6%. 1H NMR (500 MHz, DMSO-d6) δ 0.77 (t, J=7.6 Hz, 6H), 1.29-1.36(m, 2H), 1.57-1.65 (m, 2H), 6.95 (br s, 1H), 7.23 (br s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 8.2, 32.5, 60.6, 178.2 One equivalent of 1,3-propane was added to a solution of 2-amino-2-ethylpropaneamide (0.941 g, 7.23 mmol). A paste was obtained after the reaction. The paste was dissolved in water and washed with ethyl acetate. The sodium salt was prepared with 1N NaOH and the solution was concentrated to dryness. The crude product was purified by preparative RP-HPLC (Delta Prep pack cartridge C18, 215 nm, 50 mL/min, 0% to 30% MeCN in water containing 0.01% TFA). After freeze-drying, the title compound was obtained as a fine white solid (0.3700 g, 1.47 mmol, 20%). 1H NMR (300 MHz, D2O) δ 0.89 (t, J=7.5 Hz, 6H), 1.83 (q, J=7.3 Hz, 4H), 2.05 (qt, J=7.4 Hz, 4H), 2.84-2.86 (m, 2H), 2.99 (t, J=7.3 Hz, 2H); 13C NMR (75 MHz, D2O) δ 7.4, 23.5, 26.0, 41.5, 48.9, 68.3, 176.9; ES-MS 251 (M-H).
References:
US2006/223855,2006,A1 Location in patent:Page/Page column 165-166

96-22-0
275 suppliers
$9.00/1g

22374-51-2
13 suppliers
inquiry
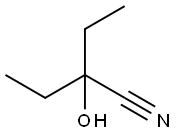
34451-66-6
14 suppliers
inquiry

22374-51-2
13 suppliers
inquiry