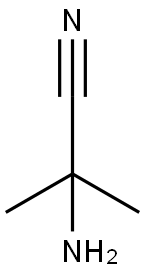
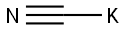2-AMINO-2-METHYL-PROPIONITRILE HYDROCHLORIDE synthesis
- Product Name:2-AMINO-2-METHYL-PROPIONITRILE HYDROCHLORIDE
- CAS Number:50846-36-1
- Molecular formula:C4H9ClN2
- Molecular Weight:120.58
19355-69-2
120 suppliers
$20.00/25 g

50846-36-1
124 suppliers
$6.00/5g
Yield:-
Reaction Conditions:
with hydrogenchloride in diethyl ether at 0; for 0.5 h;
Steps:
12.1
EXAMPLE 12 Synthesis OFNL- (L-CYANO-L-METH LEYL)-N2- (LS)-2, 2, 2-TRIFLUORO-1-R4 - (METHYLSULFONYL) BIPHENYL-4- YLLETHYL}-L-LEUCINAMIDE STEP 1 : Preparation of 2-AMINO-2-METHYLPROPANENITRILE hydrochloride; To a 0 °C solution of ammonium chloride (15.5 g) in water (50 mL) was added a solution of acetone (17 mL) in diethyl ether (50 mL). Then a solution of the sodium cyanide (11.9 g. ) in water (35 ML) was slowly added at such a rate that the temperature never exceeds 10 °C. The reaction mixture was stirred for one hour at 0 °C after the addition of the cyanide solution then it was allowed to stand overnight. The ether layer was separated and the aqueous layer was extracted with diethyl ether (2 x 30 ML). The combined organic layers were washed with brine, dried with magnesium sulfate and the solvent were removed in vacuo to yield a residue which was diluted with methyl alcohol (80 mL). The solution was cooled at-78 °C and saturated with ammonia gas (An Ace pressure tube with plunger valve and thermo well was used). The reaction mixture was allowed to stand at room temperature for two days. The excess ammonia was expelled by a current of air and the methyl alcohol was removed by evaporation. The residue was dissolved in diethyl ether (50 mL) and cooled to 0 °C then a solution of 40 mL of hydrogen chloride (1.0 M in diethyl ether) was added. The mixture was stirred for 30 minutes and filtered to yield the title compound. 1H NMR (CD3SOCD3) 8 9.45 (1H, s), 1.70 (6H, s).
References:
MERCK FROSST CANADA & CO. WO2005/21487, 2005, A1 Location in patent:Page/Page column 64-65