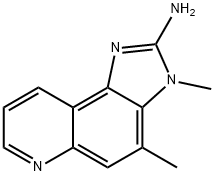
2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline synthesis
- Product Name:2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline
- CAS Number:77094-11-2
- Molecular formula:C12H12N4
- Molecular Weight:212.25

537-92-8
121 suppliers
$10.00/1g
![2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline](/CAS/GIF/77094-11-2.gif)
77094-11-2
44 suppliers
$96.00/2 mg
Yield:-
Steps:
Multi-step reaction with 7 steps
1: 75 percent / HNO3, conc. H2SO4 / 0.5 h / 0 °C
2: 1.) H2SO4, arsenic acid; 2.) H2 / 2.) 10percent Pd-C / 1.) 130 deg C, 18 h; 2.) EtOH, 7 d
3: 90 percent / (CH3CO)2O / 2 h / Ambient temperature
4: 90 percent / LiAlH4 / tetrahydrofuran / 0 °C
5: 68 percent / HNO3, H2SO4 / 1 h / 0 °C
6: 80 percent / SnCl2, HCl / 1 h / 100 °C
7: ethanol / 2 h
References:
Lee;Hashimoto;Shudo;Okamoto [Chemical and Pharmaceutical Bulletin,1982,vol. 30,# 5,p. 1857 - 1859]

83407-41-4
22 suppliers
inquiry
![2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline](/CAS/GIF/77094-11-2.gif)
77094-11-2
44 suppliers
$96.00/2 mg

51366-39-3
57 suppliers
$82.00/5g
![2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline](/CAS/GIF/77094-11-2.gif)
77094-11-2
44 suppliers
$96.00/2 mg
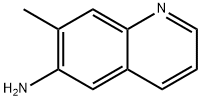
77094-10-1
4 suppliers
inquiry
![2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline](/CAS/GIF/77094-11-2.gif)
77094-11-2
44 suppliers
$96.00/2 mg

86984-28-3
19 suppliers
inquiry
![2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline](/CAS/GIF/77094-11-2.gif)
77094-11-2
44 suppliers
$96.00/2 mg