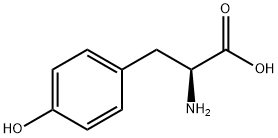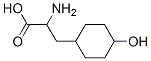
2-Amino-3-(4-hydroxycyclohexyl)propionic acid synthesis
- Product Name:2-Amino-3-(4-hydroxycyclohexyl)propionic acid
- CAS Number:4441-51-4
- Molecular formula:C9H17NO3
- Molecular Weight:187.24
Yield:-
Reaction Conditions:
with hydrogenchloride;hydrogen in water at 100; pH=1; under 37503.8 Torr; for 24 h;Autoclave;
Steps:
2.3. Catalysis
The selective hydrogenation of the optically active phenyl amino acid was carried out in a magnetically stirred stainless-steel autoclave (100 ml). Prior to the loading of the catalyst, the autoclavewas purged three times with hydrogen to expel the air. Typically, a freshly prepared suspension of nanoRu(at)hectorite (0.01592 mmol Ru, 10 ml H2O) and the appropriate amount of the substrate were carefully transferred into the autoclave under inert atmosphere, and then the autoclave was charged with H2 to the desired pressure. The autoclave was placed into the pre-heated heating mantle and the magnetic stirring was started for the indicated reaction time. After the reaction, the autoclave was cooled down and the pressure was released. The reactor was thoroughly rinsed with 2 N NaOH solution to wash out the entire product (in the case of acidic system, 2 N HCl was used). All the collected solutions were filtered (0.22 mm, PTFE) to remove the catalyst and then treated with diluted HCl (or NaOH) solution to adjust the pH to 5.5, which caused the partial precipitation of the product. The suspensionwas then reduced in vacuo to 10 ml in order to complete the precipitation. The precipitate was filtered off, washed with distilled water and dried in vacuo for 24 h.
References:
Sun, Bing;Süss-Fink, Georg [Journal of Organometallic Chemistry,2016,vol. 812,p. 81 - 86]