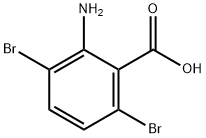
2-amino-3,6-dibromobenzoic acid synthesis
- Product Name:2-amino-3,6-dibromobenzoic acid
- CAS Number:20776-66-3
- Molecular formula:C7H5Br2NO2
- Molecular Weight:294.93
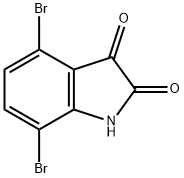
20780-89-6
6 suppliers
inquiry

20776-66-3
7 suppliers
inquiry
Yield:20776-66-3 79%
Reaction Conditions:
with dihydrogen peroxide;sodium hydroxide in water at 50; for 0.5 h;
Steps:
1
2,5- dibromo aniline (compound 1, 7.53 g, 30 mmol), Chloral monohydrate (5.95 g, 36 mmol), hydroxy amine - hydrogen chloride (3.13 g, 45 mmol) and sodium sulfate (30.0 g) were added into a mixed solvent of water (100 mL) & ethanol (100 mL). These mixture was heated-refluxed for 12 hours at 80°C. Thereafter, ethanol was removed under reduced pressure, concentrated, and when poured into crushed ice, a white solid was precipitated. As it is, allowed to stand for 3 hours at 0°C, then the precipitated white solid was recovered by filtration, and dried in air to obtain 8.28 g (86%) of 2,5- dibromo isonitroso acetoanilide (compound 2). Subsequently, this compound 2 was heated for 15 mins at 100°C in 86% sulfuric aicd (80 mL), to proceed the cyclization reaction, and the color of the reaction product changes to dark red. Here the resulting product was poured into crushed ice and 4.96 g (63%) of 3,6- dibromo isatin (compound 3) was precipitated as light orange crystals. Then, the produced compound 3 was done hydrolysis under basic conditions by adding hydrogen peroxide and 3.79 g (79%) of 3,6- dibromo Anthranilic Acid (compound 4) was obtained as Dull white crystals. Finally, compound 4 was done diazotization under the existence of nonproton, and by doing this, it is possible to change to 1,4- dibromo- 2,3- diiodo benzene (compound 5). Hexane as eluent, by silica gel column chromatography or by distillation under reduced pressure, compound 5 was obtained as white crystals with yield 35% (2.19 g).
References:
JP6132656,2017,B2 Location in patent:Paragraph 0035
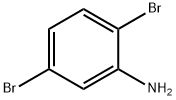
3638-73-1
202 suppliers
$10.00/1g

20776-66-3
7 suppliers
inquiry

529502-68-9
1 suppliers
inquiry

20776-66-3
7 suppliers
inquiry