
2-AMINO-3-(METHYLTHIOMETHYL)BENZOTRIFLUORIDE synthesis
- Product Name:2-AMINO-3-(METHYLTHIOMETHYL)BENZOTRIFLUORIDE
- CAS Number:88301-96-6
- Molecular formula:C9H10F3NS
- Molecular Weight:221.24
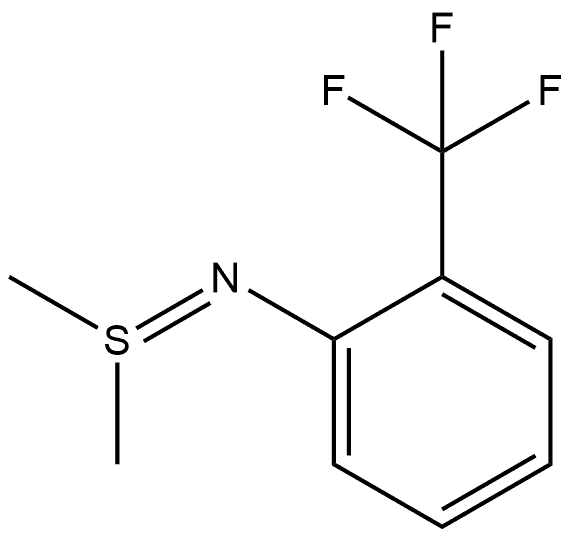
88798-15-6
0 suppliers
inquiry

88301-96-6
24 suppliers
$65.00/50mg
Yield:88301-96-6 77%
Reaction Conditions:
with Succinimide in 1,2-dichloro-ethane; for 4 h;Reflux;
Steps:
(2-Trifluoromethyl)-6-[(methylthio)methyl]aniline(3)
2-TriFluoromethyl aniline 1 (100 mL, 0.8 mol) and phosphoruspentoxide (340 g, 2.4 mol) were suspended in1600 mL CH2Cl2 and stirred at room temperature whileDMSO (400 mL, 5.6 mol) and triethylamine (78 mL,0.56 mol) were added slowly during 30 min, the reactionmixture was heated to 30 °C for 8 h. Then, the reactionmixture was poured to 200 mL 10% ice NaOH solution. N-(2-trifluoromethylphenyl)-S, S-dimethyl sulfilimine (2,104.0 g) was obtained by separated CH2Cl2 layer, driedwith anhydrous sodium sulfate and removed solvent byrotaryevaporation. Compound 2 (104 g, 0.47 mol) and succinimide (2.3 g, 0.023 mol) were then added to 600 mL1, 2-dichloroethane, the mixture was heated to reflux for4 h. The reaction mixture was poured to 200 mL 10% iceNaOH solution. 2-Trifluoromethyl-6-[(methylthio)methyl]aniline (3, 80 g) was obtained by separated 1,2-dichloroethanelayer, dried with anhydrous sodium sulfate andremoved solvent by rotaryevaporation. Yield 77%.
References:
Li, Wen;Yan, Ying;Chang, Yingjie;Ding, Lina;Liu, Hongmin;You, Qidong [Medicinal Chemistry Research,2019,vol. 28,# 10,p. 1783 - 1795]

75-18-3
389 suppliers
$19.00/25mL
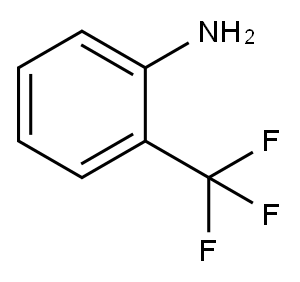
88-17-5
364 suppliers
$14.00/25g

88301-96-6
24 suppliers
$65.00/50mg

88-17-5
364 suppliers
$14.00/25g

88301-96-6
24 suppliers
$65.00/50mg