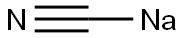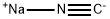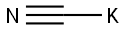2-AMINO-3-PHENYLPROPIONITRILE HYDROCHLORIDE synthesis
- Product Name:2-AMINO-3-PHENYLPROPIONITRILE HYDROCHLORIDE
- CAS Number:93554-83-7
- Molecular formula:C9H11ClN2
- Molecular Weight:182.65
Yield:93554-83-7 80%
Reaction Conditions:
Stage #1:sodium cyanide with ammonia in water at 25; under 1125.11 Torr; for 0.5 h;
Stage #2:phenylacetaldehyde with acetic acid in water at 35; for 4.75 h;
Stage #3: with hydrogenchloride in 1,4-dioxane;diethyl ether
Steps:
1
Example 1: Strecker synthesis of rac-2-amino-3-phenylpropionitrile A 30 wt% solution of sodium cyanide (653 g, 4.0 mol) at 25§C was saturated with ammonia gas at 0.15 MPa In 30 min time 240 g (4.0 mol) of acetic acid was added, followed by the addition of 534 g (4.0 mol) of phenylacetaldehyde (80-90%) in 45 min. After stirring for 4 h at 35§C, the reaction mixture was extracted with 2 L of CH2Cl2. The organic layer was evaporated and the residue dissolved in 4.5 L of diethyl ether. To this solution 3-3.5 mol of saturated HCI in dioxan was added. The precipitated rac-2-amino-3-phenylpropionitrile.HCI was filtered and washed with ether (yield 80-85%).
References:
DSM IP Assets B.V. EP1352894, 2003, A1 Location in patent:Page/Page column 4
![Benzenepropanenitrile, α-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/93554-96-2.gif)
93554-96-2
0 suppliers
inquiry

93554-83-7
24 suppliers
inquiry
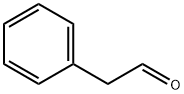
122-78-1
311 suppliers
$10.00/5g

93554-83-7
24 suppliers
inquiry