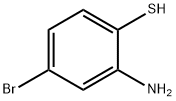
2-AMINO-4-BROMOBENZENETHIOL synthesis
- Product Name:2-AMINO-4-BROMOBENZENETHIOL
- CAS Number:93933-49-4
- Molecular formula:C6H6BrNS
- Molecular Weight:204.09
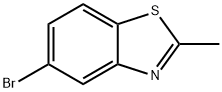
63837-11-6
156 suppliers
$15.00/1g

93933-49-4
32 suppliers
inquiry
Yield: 96%
Reaction Conditions:
with water;sodium hydroxide in ethylene glycol at 140; for 4 h;
Steps:
23.1 Step1: 2 -Amino-4-bromobenzenethiol
To a stirring solution of 5-bromo-2-methylbenzo[djthiazole (5 g, 22.02 mmol) in ethylene glycol (50 mL) was added 8N sodium hydroxide solution (50 mL) and the resulting reaction mixture was stirred at 140 °C for 4 h. Progress of the reaction was monitored by TLC and LCMS. After completion, the reaction mixture was concentrated under reduced pressure to get a crude residue, which was purified by triturating it with pentane/ether to afford the title compound as a yellow solid (4.3 g, 96% yield). ‘H NMR (400 MHz, DMSO-d6) = 7.09 (d, J=8.33 Hz, 1H), 6.87 (d, J=1.75 Hz, 1H), 6.62 (dd, J=1.75, 8.33 Hz, 1H), 5.16 (br. s, 3H). LCMS: [M+Hj = 203.83; R = 3.10 mm.
References:
SYROS PHARMACEUTICALS, INC.;ROBERTS, Christopher;ZHANG, Yi;BEAUMIER, Francis;LEPISSIER, Luce;MARINEAU, Jason, J.;RAHL, Peter, B.;SPROTT, Kevin;CIBLAT, Stephane;SOW, Boubacar;LAROUCHE-GAUTHIER, Robin;BERSTLER, Lauren WO2016/197078, 2016, A1 Location in patent:Paragraph 395; 396-397; 565; 566-567; 935-936; 950-951; 1010
76209-05-7
0 suppliers
inquiry
76209-02-4
15 suppliers
inquiry
![2-[(2-AMino-4-broMophenyl)disulfanyl]-5-broMoaniline](/CAS/20150408/GIF/7038-31-5.gif)
7038-31-5
7 suppliers
$85.00/50mg

93933-49-4
32 suppliers
inquiry
3460-18-2
287 suppliers
$6.00/10g

93933-49-4
32 suppliers
inquiry

76209-02-4
15 suppliers
inquiry

93933-49-4
32 suppliers
inquiry
20358-03-6
143 suppliers
$8.00/250mg

93933-49-4
32 suppliers
inquiry