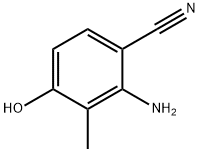
2-aMino-4-hydroxy-3-Methylbenzonitrile synthesis
- Product Name:2-aMino-4-hydroxy-3-Methylbenzonitrile
- CAS Number:102569-26-6
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
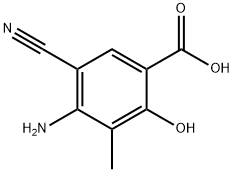
72817-94-8
18 suppliers
inquiry

102569-26-6
10 suppliers
inquiry
Yield:102569-26-6 82%
Reaction Conditions:
with quinoline at 170; for 2 h;
Steps:
39.C
C: Synthesis of 2-Amino-4-hydroxy-3-methylbenzonitrile (143); A mixture of compound 75 (19 g, 0.1 mol) in quinoline (50 mL) was heated to 170 °C for 2h (until effervescence ceased). The reaction mixture was cooled to RT and aqueous NaOH solution was added (IM, 500 mL) followed by pet. ether (500 mL). The reaction mixture was stirred for 15 min and the aqueous layer was separated. The aqueous layer was further washed with pet. ether (2x300 mL) to remove quinoline completely. The aqueous layer was acidified with 1.5N HCl to pH=5, the solid was filtered off and dried under vacuum. The obtained solid was further washed with 5% ethyl acetate in pet. ether to give pure title compound (12 g, 82%). TLC: EIOAc/ Pet ether, 3:7, R^0.35
References:
WO2007/14926,2007,A1 Location in patent:Page/Page column 128
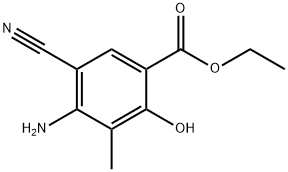
72817-85-7
23 suppliers
inquiry

102569-26-6
10 suppliers
inquiry