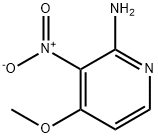
2-AMINO-4-METHOXY-3-NITROPYRIDINE synthesis
- Product Name:2-AMINO-4-METHOXY-3-NITROPYRIDINE
- CAS Number:84487-08-1
- Molecular formula:C6H7N3O3
- Molecular Weight:169.14

67-56-1
739 suppliers
$9.00/25ml

124-41-4
682 suppliers
$12.00/25g
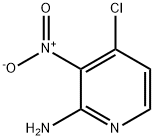
6980-08-1
186 suppliers
$15.00/1g

84487-08-1
53 suppliers
$120.00/25mg
Yield:84487-08-1 180 mg
Reaction Conditions:
Stage #1: methanol;sodium methylate;4-chloro-3-nitro-2-pyridinamine at 60; for 16 h;
Stage #2: with hydrogenchloride in water; pH=6;
Steps:
(8-Methoxy-2-methyl-pyrido[2,3-b]pyrazin-3-yl)-hydrazine (He).
To a solution of 4- chloro-pyridin-2-ylamine (5 g) in 96% aq H2SO4 (20 mL) at 0 °C was added a mixture solution of 70% aq HN03 (2.5 mL) and 96% aq H2S04 (10 mL) drop-wise. After the addition was completed, the mixture was stirred at room temperature for 2h. The solution was poured onto ice/water and 6M aq. NaOH was added drop-wise to adjust pH to 9. Then the solid was filtered off, washed with water, and dried before it was purified by chromatography on alumina (eluent: pentane:EtOAc 2:1) to afford 4-chloro-3-nitro-pyridin-2-ylamine (1.2 g). 200 mg of this material was dissolved methanol (3mL) and NaOMe (125 mg) was added before the mixture was stirred at 60 °C for 16h. 37%> aq HC1 was added drop-wise to adjust pH to 6. The mixture was cooled to 0 °C, and the precipitated solid was filtered off, washed with water, and dried to afford 4-methoxy-3-nitro-pyridin-2-ylamine (180 mg). This material was dissolved in methanol (20 mL) and treated with hydrogen gas (1 bar) in the presence of 10%> palladium on charcoal at room temperature for 2h. The mixture was filtered and the filtrate was concentrated in vacuo to afford 4-methoxy-pyridine-2,3-diamine (50 mg). This procedure was repeated to produce more material. In the next step, 4-methoxy-pyridine-2,3-diamine (6.8 g) was dissolved in ethanol (200 mL). To this solution was slowly added methyl pyruvate (4.9 mL) at room temperature, and the mixture was stirred at ambient temperature for 3h before the volatiles were removed in vacuo. The residue was purified by chromatography on silica gel (eluent: pentane:EtOAc 5: 1 to 0: 1) to give afford 8-methoxy-2-methyl-4H-pyrido[2,3- b]pyrazin-3-one (6.1 g). To a suspension of 3 g of this material in DMF (12 mL) was added PyBroP (7.65 g) followed by DBU (3.58 g). The mixture was stirred overnight before the precipitated solid was filtered off, washed with ethanol, and dried to afford 3-(benzotriazol-l- yloxy)-8-methoxy-2-methyl-pyrido[2,3-b]pyrazine (3.5 g). 1.5 g of this material was dissolved in a mixture of ethanol (lOmL) and DCM (60mL). Hydrazine monohydrate (3.6g) was added, and the mixture was stirred overnight at ambient temperature before the volatiles were removed in vacuo. The residual solid was triturated from ethanol, filtered off, washed with ethanol, and dried to afford (8-methoxy-2-methyl-pyrido[2,3-b]pyrazin-3-yl)- hydrazine He (0.9 g).
References:
WO2013/34761,2013,A1 Location in patent:Page/Page column 19

124-41-4
682 suppliers
$12.00/25g

6980-08-1
186 suppliers
$15.00/1g

84487-08-1
53 suppliers
$120.00/25mg

124-41-4
682 suppliers
$12.00/25g

84487-10-5
66 suppliers
$40.00/100mg

84487-08-1
53 suppliers
$120.00/25mg
84249-01-4
0 suppliers
inquiry

84487-08-1
53 suppliers
$120.00/25mg

67-56-1
739 suppliers
$9.00/25ml

6980-08-1
186 suppliers
$15.00/1g

84487-08-1
53 suppliers
$120.00/25mg