
2-AMINO-5-(4-FLUOROBENZYL)-1,3,4-THIADIAZOLE synthesis
- Product Name:2-AMINO-5-(4-FLUOROBENZYL)-1,3,4-THIADIAZOLE
- CAS Number:39181-55-0
- Molecular formula:C9H8FN3S
- Molecular Weight:209.24
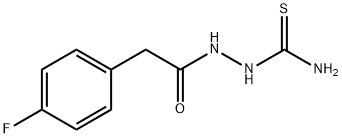
39203-09-3
1 suppliers
inquiry

39181-55-0
24 suppliers
$90.00/100mg
Yield: 0.264 g
Reaction Conditions:
Stage #1:2-[(4-fluorophenyl)acetyl]hydrazinecarbothioamide with sulfuric acid at -10 - 0; for 3 h;
Stage #2: with sodium hydroxide in water at -10 - 0;
Steps:
1.2 1.2 Synthesis of 5-(4-fluorobenzyl)-1,3,4-thiadiazol-2-amine
[0256] 5 mL of sulfuric acid are placed in a round-bottomed flask, which is cooled to between 0 and -10° C. by placing it in a bath of ice and sodium chloride. 0.38 g of 2-[(4-fluorophenyl)acetyl]hydrazinecarbothioamide (1.67 mmol) is added portionwise with stirring. After stirring for 3 hours between 0 and -10° C., water is added dropwise and the mixture is then returned to basic pH with sodium hydroxide, while maintaining a temperature of between 0 and -10° C. The precipitate is filtered off, washed with water and dried. 0.264 g of 5-(4-fluorobenzyl)-1,3,4-thiadiazol-2-amine is obtained. [0257] M+H+=210.
References:
SANOFI;Fett, Eykmar;Mougenot, Patrick;Namane, Claudie;Nicolai, Eric;Philippo, Christophe US2012/40984, 2012, A1 Location in patent:Paragraph 0256; 0257

79-19-6
505 suppliers
$14.00/1g
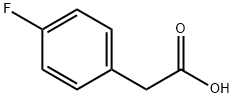
405-50-5
440 suppliers
$5.00/10g

39181-55-0
24 suppliers
$90.00/100mg

405-50-5
440 suppliers
$5.00/10g

39181-55-0
24 suppliers
$90.00/100mg