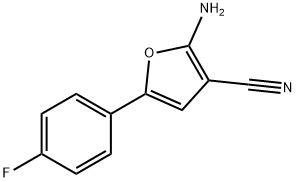
2-AMINO-5-(4-FLUOROPHENYL)FURAN-3-CARBONITRILE synthesis
- Product Name:2-AMINO-5-(4-FLUOROPHENYL)FURAN-3-CARBONITRILE
- CAS Number:708976-50-5
- Molecular formula:C11H7FN2O
- Molecular Weight:202.18
Yield:708976-50-5 63%
Reaction Conditions:
with diethylamine in N,N-dimethyl-formamide at 0 - 22; for 5.16667 h;
Steps:
4.7. General procedure synthesis of 2-amino-5-arylfurane-3-carboxynitriles (3)
General procedure: A solution of bromoketone (6-25 g) and malononitrile (1.3eq.) was stirred at 0° C in DMF (6 mL/g) for 10 min. Diethylamine(2.2 eq) was added at 0 °C over 30 min using a syringe pump andthemixturewas stirred at 22° C for 4.5 h. The solutionwas pouredonto water and left standing for 24 h at 0 °C. The formed precipitatewas filtered off, washed with water (2 x 50 mL) andpetroleum ether (50 mL) and dried in vacuo to yield targetcompound.
References:
Han, Jin;Kaspersen, Svein Jacob;Nervik, Sondre;N?rsett, Kristin G.;Sundby, Eirik;Hoff, B?rd Helge [European Journal of Medicinal Chemistry,2016,vol. 119,p. 278 - 299]
748810-25-5
13 suppliers
inquiry

708976-50-5
8 suppliers
inquiry
403-29-2
319 suppliers
$8.00/5g

708976-50-5
8 suppliers
inquiry
