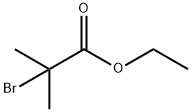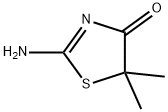
2-amino-5,5-dimethyl-1,3-thiazol-4-one synthesis
- Product Name:2-amino-5,5-dimethyl-1,3-thiazol-4-one
- CAS Number:4695-19-6
- Molecular formula:C5H8N2OS
- Molecular Weight:144.19
Yield:4695-19-6 85%
Reaction Conditions:
with sodium acetate in ethanol; for 12 h;
Steps:
1.1 step one
Ethyl 2-bromoisobutyrate (80 g, 410 mmol),Thiourea (49.95g, 656mmol),Anhydrous sodium acetate (47.10g, 574mmol) in a 1000mL three-neck bottle,The measuring cylinder takes 300mL of absolute ethanol and pours into the three-necked bottle; the left, middle and right three ports of the three-necked bottle are respectively connected with a spherical condenser, a mechanical agitator, a thermometer with a range greater than 100 °C, and the three-necked bottle is placed in a constant temperature oil bath for magnetic stirring. In the pot, the temperature is raised until the temperature indicated by the thermometer no longer rises (85 ° C), and the reaction is carried out for 12 hours. After the reaction is completed, the three-necked bottle is placed in a low-temperature water bath of about 5 ° C and cooled to room temperature of about 45 ° C;Pour the reaction solution in the three-necked bottle into a single-mouth bottle, and rinse all the residue in the three-necked bottle into a 1000-mL single-mouth bottle with ethanol. Set the temperature of the rotary evaporator to 45 ° C and set the rotation speed to 70 to connect the single-mouth bottle. To the above, remove the ethanol in the reaction solution;Dissolve sodium bromide, unreacted thiourea, and sodium acetate in the residue with 180 mL of distilled water.Until fully dissolved; use a sand core filter funnel for filtration,Obtaining a white solid 5,5-dimethyl-2-imino-4-thiazolidinone and drying it.50g (yield 85%, purity 99.54%),Called compound C.
References:
CN110128309,2019,A Location in patent:Paragraph 0025

17356-08-0
2 suppliers
inquiry
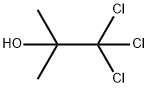
57-15-8
271 suppliers
inquiry
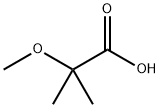
13836-62-9
68 suppliers
$75.00/1g

4695-19-6
7 suppliers
inquiry

67-56-1
790 suppliers
$9.00/25ml

57-15-8
271 suppliers
inquiry

13836-62-9
68 suppliers
$75.00/1g

4695-19-6
7 suppliers
inquiry