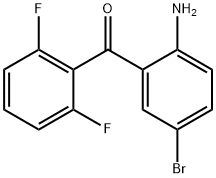
2-Amino-5-bromo-2',6'-difluoro benzophenone synthesis
- Product Name:2-Amino-5-bromo-2',6'-difluoro benzophenone
- CAS Number:660450-79-3
- Molecular formula:C13H8BrF2NO
- Molecular Weight:312.11
Yield:-
Reaction Conditions:
Stage #1: 2,6-difluorobenzoylchloride;4-bromo-anilinewith zinc(II) chloride at 120 - 205; for 3 h;
Stage #2: with hydrogenchloride in water; for 0.5 h;Heating / reflux;
Stage #3: with hydrogenchloride in water;acetic acid at 120; for 12 h;Heating / reflux;
Steps:
77.1 Example 77; methyl [3- (AMINOMETHYL)-4- (2,] 6-difluorophenyl) -2- isobutylquinoline-6-carboxylate
1) 4-Bromoaniline (14.9 g, 86.5 mmol) was heated to [120°C] and 2,6-difluorobenzoyl chloride (36.7 g, 208 mmol) was added. The temperature of the mixture was raised to [180°C] and zinc chloride (14.2 g, 104 mmol) was added. The temperature of the mixture was raised to [205°C] and the mixture was stirred for 3 hrs. The reaction mixture was cooled to [150°C,] and 4N hydrochloric acid (100 ml) was added, and the mixture was heated under reflux for 15 min. The supernatant was removed, 4N hydrochloric acid (100 ml) was added to the residue, and the mixture was heated under reflux for 15 min. The supernatant was removed and the residue was dissolved in acetic acid (85 ml). [CONC.] hydrochloric acid (85 ml) was added at [120°C] and the mixture was heated under reflux for 12 hrs. The reaction mixture was concentrated under reduced pressure and 1N aqueous sodium hydroxide solution (200 ml) was-added to the residue. The mixture was extracted with ethyl acetate, and the extract was washed with 1N hydrochloric acid and saturated brine and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography to give a crude product of (2-amino-5-bromophenyl) (2,6- difluorophenyl) methanone (7.8 [G).] To a solution of the crude product (5.2 g) and 5-methyl-3-oxohexanenitrile (2.1 g, 17 mmol) in toluene (100 ml) was added. methanesulfonic acid (1.6 g, 17 mmol) and the mixture was heated under reflux for 17 hrs. The reaction mixture was washed with saturated aqueous sodium hydrogen carbonate and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography to give [6-BROMO-4- (2,] 6-difluorophenyl) - 2-isobutylquinoline-3-carbonitrile (1.6 g, yield 4. [6%)] as a yellow solid. [LH-NMR] [(CDC13) USD : 1. 06] (6H, d, J = 6.6 Hz), 2.30-2. 50 (1H, m), 3.12 (2H, d, J [= 7.] 4 Hz), 7.15-7. 25 (2H, m), 7.55-7. 70 (2H, m), 7.91 [(1H,] dd, J = 2.1, 9.1 Hz), 8.03 [(1H,] d, J = 9.1 Hz).
References:
WO2004/14860,2004,A2 Location in patent:Page 175-176

