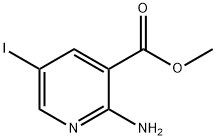
2-AMino-5-iodo-nicotinic acid Methyl ester synthesis
- Product Name:2-AMino-5-iodo-nicotinic acid Methyl ester
- CAS Number:1227048-78-3
- Molecular formula:C7H7IN2O2
- Molecular Weight:278.05
14667-47-1
205 suppliers
$6.00/1g

1227048-78-3
52 suppliers
$25.00/100mg
Yield:-
Reaction Conditions:
with N-iodo-succinimide in N,N-dimethyl-formamide; for 16 h;
Steps:
76
Preparation 76: 2-Amino-5-(4-fluorophenoxy)nicotinic add methyl ester To 2-aminonicotimc acid methyl eater (5.5g, 36.2mmol) in DMF (70mL) was added N-iodosuccinimide (9.8g, 43.7rmnol). After 16h the mixture was poured into sat. sodium thiosulfite solution and then extracted with Et20. The organic phase was washed with water, brine, dried (MgS04) and the solvent was removed in vacuo to give 2-amino-5-iodonicotinic acid methyl ester. To 4-fluorophenol (2.4g, 21.6mmol) in dioxane (50mL) was addedCS2CO3 (6g, 25.2mmol) and the mixture was heated to 50°C. After 20min Cul (0.56g, 3.0mmol) and 2-ammo-5-iodonicotrriic acid methyl ester (2g, 7.2mmol) were added and the mixture heated under reflux for 16h. After cooling the solvent removed in vacuo, the residue was partitioned between EtOAc and 4N HQ, the organic phase was extracted with 6N HC1 and the organic phase discarded. The combined aqueous phase was basified with NH4OH and re-extracted with EtOAc. The organic phase was dried (MgS04) and the solvent was removed in vacuo. The residue was purified by column chromatography (1 :3EtOAcrHexane) to give, after removal of the solvent in vacuo, the title compound: RT= 3.30min; m/z (ES+) = 263.2 [M+ Iff".
References:
WO2011/117254,2011,A1 Location in patent:Page/Page column 52