2-AMINO-5-METHYL-4,5,6-TRIHYDROBENZOTHIAZOL-7-ONE synthesis
- Product Name:2-AMINO-5-METHYL-4,5,6-TRIHYDROBENZOTHIAZOL-7-ONE
- CAS Number:375825-03-9
- Molecular formula:C8H10N2OS
- Molecular Weight:182.24
4341-24-6
177 suppliers
$8.00/1g

17356-08-0
3 suppliers
inquiry

375825-03-9
17 suppliers
$52.50/250mg
Yield:375825-03-9 86%
Reaction Conditions:
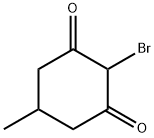
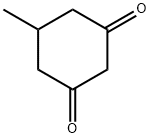
Stage #1: 5-methylcyclohexan-1,3-dionewith bromine;sodium acetate;acetic acid at 15 - 20;
Stage #2: thiourea at 100; for 1 h;
Steps:
A.04.B
5-Methyl-cyclohexane- 1,3-dione (70.0 g, 0.555 mol) and NaOAc (68.3 g, 0.832 mol) are suspended in AcOH (700 mL) and Br2 (88.7 g, 0.555 mol) is added drop-wise while maintaining the reaction temperature between 15 and 20 0C. After complete addition, the reaction is stirred at RT overnight. Thiourea (42.2 g, 0.555 mol) is added in portions and the reaction mixture is heated to 100 0C for 1 h. After cooling to RT, the AcOH is removed in vacuo. The resulting crude product is diluted with water (1 L), adjusted to pH = 7 with NaHCO3 solution (3 M) and the resulting mixture is extracted with EtOAc (3 x 1 L). The organic phase is washed with brine and dried over anhydrous Na2SO4. The solvent is evaporated in vacuo and the residue is recrystallized from hexane to give 2-amino-5- methyl-5,6-dihydro-4H-benzothiazol-7-one (87.2 g, 86 % yield).1H NMR: (400 MHz, DMSO-J6) δ 8.09 (br s, 2H), 2.77-2.72 (m, IH), 2.39-2.13 (m, 4H), 1.03 (d, J= 6.0 Hz, 3H).
References:
WO2010/122091,2010,A1 Location in patent:Page/Page column 15

4341-24-6
177 suppliers
$8.00/1g

375825-03-9
17 suppliers
$52.50/250mg