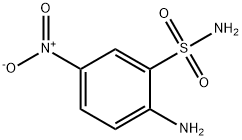
2-aMino-5-nitrobenzene-1-sulfonaMide synthesis
- Product Name:2-aMino-5-nitrobenzene-1-sulfonaMide
- CAS Number:54734-85-9
- Molecular formula:C6H7N3O4S
- Molecular Weight:217.2

20628-33-5
2 suppliers
inquiry

54734-85-9
18 suppliers
inquiry
Yield: 203.8 g
Reaction Conditions:
with ammonium hydroxide in sulfolane;water;acetonitrile at 20; for 2 h;
Steps:
1 Step 1: Sulfonamide Formation
1. Phosphorus oxychloride (316 g, 192 mL, 2.06 mol, 1.8 equivalents) was slowly added to a suspension of 2-amino-5-nitrobenzenesulfonic acid (250 g, 1.15 mol, 1.00 equivalent) in a mixture of acetonitrile (2.54 kg, 2.0 L, 8 vol) and sulfolane (254 g, 200 mL, 0.8 vol) at ambient temperature. [0053] 2. This mixture was stirred at 75-80° C. for 3-5 h, and then the supernatant was slowly transferred to NH4OH solution (28-30%, 1.5 L, 6 vol) such that the temperature was maintained below 30° C. [0054] 3. The reaction mixture was stirred at ambient temperature ca. 2 h, and was then concentrated under reduced pressure at ca. 50° C. to a minimum volume to obtain a slurry. [0055] 4. This slurry was aged at ca. 50° C. for ca. 2 h and then slowly cooled to ca. 10° C. and aged at ca. 10° C. for ca. 2 h. The solid was filtered and washed with water (3×250 mL, 3×1 vol) to give a yellow product which was dried at ca. 50° C. under vacuum for 2 days to yield 203.8 g of II (2-amino-5-nitro-benzenesulfonamide, See also compound described in Dragovich, P. S.; Thompson, P. A; Reubsam, F. WO 2010/042834, herein incorporated by reference in its entirety) in 81.9% yield with 99.0% purity by AN HPLC (area normalized high performance liquid chromatography).
References:
Hoffmann-La Roche Inc.;Zhu, Jiang US2014/200362, 2014, A1 Location in patent:Paragraph 0051-0055
96-72-0
105 suppliers
$16.00/1G

54734-85-9
18 suppliers
inquiry

96-75-3
158 suppliers
$13.00/25g

54734-85-9
18 suppliers
inquiry

13338-00-6
10 suppliers
inquiry

54734-85-9
18 suppliers
inquiry

30693-53-9
95 suppliers
$10.00/1g

54734-85-9
18 suppliers
inquiry