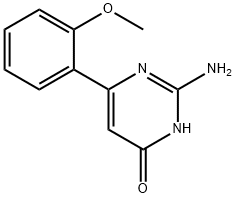
2-AMINO-6-(2-METHOXYPHENYL)PYRIMIDIN-4-OL synthesis
- Product Name:2-AMINO-6-(2-METHOXYPHENYL)PYRIMIDIN-4-OL
- CAS Number:55982-12-2
- Molecular formula:C11H11N3O2
- Molecular Weight:217.22
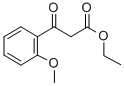
41607-95-8
65 suppliers
$27.00/100mg
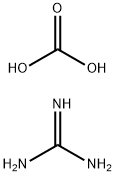
593-85-1
479 suppliers
$6.00/25g

55982-12-2
4 suppliers
inquiry
Yield:55982-12-2 72%
Reaction Conditions:
in 2,2,2-trifluoroethanol at 80;
Steps:
General Procedure 2: Trifluoroethanol-mediated guanidine condensation (isolation by precipitation)
General procedure: The β-keto ester (2 mmol) and guanidine carbonate (360 mg, 2 mmol) were weighed into a vial containing a stir bar. Trifluoroethanol(2.5 mL) was added and the vial was placed in a heating block that had been preheated to 80 °C. The mixture stirred at that temperature until the starting material had been consumed as determined by LC-MS (typically < 16 hours). The reaction mixture was allowed to cool to room temperature, and solvent was removed under reduced pressure to obtain a viscous red oil. The oil was re-dissolved in water (2.5mL) and extracted with dichloromethane (2 x 2.5 mL). The aqueous phase was then acidified to pH ~2-3 using 1M HCl. The precipitated product was collected by filtration, washed with water, and dried in vacuo to yield the pyrimidinone product.
References:
Rosen, Brandon R.;Ul Sharif, Ehesan;Miles, Dillon H.;Chan, Nicholas S.;Leleti, Manmohan R.;Powers, Jay P. [Tetrahedron Letters,2020,vol. 61,# 20,art. no. 151855] Location in patent:supporting information

41607-95-8
65 suppliers
$27.00/100mg

113-00-8
61 suppliers
$909.55/5G

55982-12-2
4 suppliers
inquiry