
(2-AMINO-6-BROMOPYRIDIN-3-YL)METHANOL synthesis
- Product Name:(2-AMINO-6-BROMOPYRIDIN-3-YL)METHANOL
- CAS Number:1620239-70-4
- Molecular formula:C6H7BrN2O
- Molecular Weight:203.04
1196157-51-3
60 suppliers
$42.00/100mg

1620239-70-4
7 suppliers
inquiry
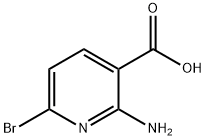
Yield:1620239-70-4 1.55 g
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether at 0 - 20;Inert atmosphere;
Steps:
1 Step 1: (2-Amino-6-bromopyridin-3-yl)methanol (2-S2)
To 2-amino-6-bromonicotinic acid (2-Si, 2 g, 9.22 mmol) in THF (70 mL), LAH (1M in ether, 18.4 mL, 18.4 mmol) was added slowly at 0 °C under argon with stirring. After completion of the addition, the ice bath was removed and the mixture was stirred at room temperature overnight. The reaction mixture was cooled again in an ice bath and saturated NH4C1 aqueous solution (25 mL) was added with stirring to form a slurry. The organic layer was separated by decantation and the slurry was washed with EtOAc. The combined organic layers were washed with iN NaOH aqueous solution and brine and dried over anhydrous Na2SO4. Solvent was removed under reduced pressure to afford (2-amino-6-bromopyridin-3-yl)methanol, (2-S2, 1.55 g)as a solid.
References:
WO2018/160892,2018,A1 Location in patent:Paragraph 0871; 0872