
2-AMINO-6-CHLORO-4-(TRIFLUOROMETHYL)PHENOL synthesis
- Product Name:2-AMINO-6-CHLORO-4-(TRIFLUOROMETHYL)PHENOL
- CAS Number:78068-81-2
- Molecular formula:C7H5ClF3NO
- Molecular Weight:211.57
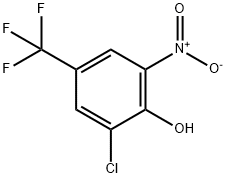
69741-64-6
10 suppliers
inquiry

78068-81-2
33 suppliers
$60.00/5mg
Yield:-
Reaction Conditions:
with hydrogen;5% Pd(II)/C(eggshell) in ethyl acetate at 20; for 15 h;
Steps:
27
Reference Production Example 27To a mixture of 3.78 g of 2-chloro-4-(trifluoromethyl)phenol, 12 ml of acetic acid and 3 ml of concentrated sulfuric acid, a mixture of 21.5 g of 69% nitric acid and 2 ml of acetic acid was added while ice-cooling. The reaction mixture was stirred while heating at room temperature for 30 minutes and then at 60° C. for two hours. After the reaction mixture was cooled to room temperature, then the reaction mixture was poured into water, and extracted with ethyl acetate three times. The combined organic layers were washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was subjected to silica gel column chromatography to give 5.01 g of a mixture containing 2-chloro-6-nitro-4-(trifluoromethyl)phenol.1H-NMR (CDCl3) δ: 11.26 (br s, 1H), 8.36 (m, 1H), 7.95 (d, J=2.2 Hz, 1H)A mixture of 5.01 g of a mixture containing 2-chloro-4-(trifluoromethyl)-6-nitrophenol, 15 ml of ethyl acetate and 1.0 g of 5% palladium on carbon was stirred under about one atmosphere of hydrogen at room temperature for 15 hours. The mixture was filtered through Celite. The filtrate was concentrated under reduced pressure. The residue was subjected to silica gel column chromatography to give 2.78 g of 2-amino-6-chloro-4-(trifluoromethyl)phenol.1H-NMR (CDCl3) δ: 7.00 (m, 1H), 6.84 (d, J=2.2 Hz, 1H), 5.80 (br s, 1H), 4.05 (br s, 2H)To a mixture of 0.58 g of WSC and 5 ml pyridine, 0.37 g of isonicotinic acid was added. The reaction mixture was stirred at room temperature for 15 minutes. To the reaction mixture, 0.63 g of 2-amino-6-chloro-4-(trifluoromethyl)phenol that had been obtained in the above-mentioned reaction was added. The reaction mixture was stirred while heating at 60° C. for three hours. The reaction mixture was cooled to room temperature, and then concentrated under reduced pressure. Water was added to the residue, followed by extraction with ethyl acetate twice. The combined organic layers were washed with a saturated sodium chloride solution, then dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was washed with a mixture solvent of tert-butyl methyl ether and hexane to give 0.42 g of N-[3-chloro-5-(trifluoromethyl)-2-hydroxyphenyl]isonicotinamide.1H-NMR (DMSO-d6) δ: 10.27 (br s, 1H), 8.81-8.79 (m, 2H), 7.90-7.88 (m, 2H), 7.86 (d, J=2.0 Hz, 1H), 7.68-7.67 (m, 1H)
References:
US2011/39843,2011,A1 Location in patent:Page/Page column 83

35852-58-5
145 suppliers
$14.00/5g

78068-81-2
33 suppliers
$60.00/5mg