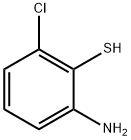
2-amino-6-chlorobenzene-1-thiol synthesis
- Product Name:2-amino-6-chlorobenzene-1-thiol
- CAS Number:14482-33-8
- Molecular formula:C6H6ClNS
- Molecular Weight:159.64
1849-73-6
62 suppliers
$9.00/100mg

14482-33-8
5 suppliers
inquiry
Yield:14482-33-8 30%
Reaction Conditions:
with hydrazine hydrate
Steps:
Compound 12 (807mg, 4.0mmol) was dissolved in hydrazine hydrate (20mL) and the mixture stirred at 110°C for 16h. After the reaction was complete, the mixture was diluted with H2O (30mL) and the pH was adjusted to 7 with 10% HCl. The aqueous phase was extracted with EtOAc (2×50mL) and the organic layer subsequently washed with brine (20mL), dried with MgSO4, and evaporated. The product, 2-amino-6-chlorobenzenethiol (XXI), was purified by reversed phase flash column chromatography using eluents A (0.1% HCOOH in MeCN) and B (0.1% HCOOH in H2O) (gradient from 1:9 to 10:0). Yellow solid (194mg, 30% yield). According to LC-MS, the product contained desired thiol compound XXI, but also the in situ formed disulfide in a substantial amount (38%); therefore, it was transferred to the next reaction step immediately.
References:
Kollár, Levente;Gobec, Martina;Szilágyi, Bence;Proj, Matic;Knez, Damijan;ábrányi-Balogh, Péter;Petri, László;Imre, Tímea;Bajusz, Dávid;Ferenczy, Gy?rgy G.;Gobec, Stanislav;Keser?, Gy?rgy M.;Sosi?, Izidor [European Journal of Medicinal Chemistry,2021,vol. 219,art. no. 113455] Location in patent:supporting information

1849-73-6
62 suppliers
$9.00/100mg

14482-33-8
5 suppliers
inquiry
608-27-5
234 suppliers
$13.00/5g

14482-33-8
5 suppliers
inquiry