
2-AMINO-6-NITROBENZONITRILE synthesis
- Product Name:2-AMINO-6-NITROBENZONITRILE
- CAS Number:63365-23-1
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
Yield:49635-80-5 13% ,63365-23-1 18%
Reaction Conditions:
with hydrogenchloride;methanol;water;iron in 1,4-dioxane at 64 - 75; for 1 h;
Steps:
1 Step 1: 2,6-diaminobenzonitrile
2,6-Dinitrobenzonitrile (3 g, 15.5 mmol, Eq: 1.0) was dissolved in a mixture of methanol (60 mL) and dioxane (35 mL). The reaction was heated to 75 °C, then HCl (37% aq, 11 g, 9.29 mL, 111 mmol, Eq: 7.16) was added dropwise, then iron (2.78 g, 49.7 mmol, Eq: 3.2) was added in 4 portions over 8 min. The reaction mixture was stirred for 1 h at 64 °C, then concentrated in vacuo. The residue was taken up in 2-methyl-THF and ice and washed with sat. aq. NaHCO3. Both layers were filtered, and the aqueous layer was back-extracted with 2-methyl-THF. The organic layers were combined, washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was diluted with DCM, evaporated with silica gel to dryness and transferred to a column. Purification by flash chromatography (80 g silica, DCM) gave the title compound (268 mg, 13% yield) along with 2-amino-6-nitro-benzonitrile (465 mg, 18% yield).2,6-diaminobenzonitrile: 1H NMR (300 MHz, DMSO-d6) δ ppm 5.55 (s, 4 H) 5.89 (d, J=8.1 Hz, 2 H) 6.89 (t, J=8.1 Hz, 1 H). 2-amino-6-nitro-benzonitrile: 1H NMR (300 MHz, DMSO-d6) δ ppm 6.74 (br s, 2 H) 7.20 (dd, J=8.3, 1.0 Hz, 1 H) 7.40 - 7.55 (m, 2 H).
References:
WO2021/116050,2021,A1 Location in patent:Page/Page column 38-39
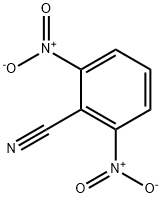
35213-00-4
58 suppliers
$50.00/2g

63365-23-1
28 suppliers
inquiry
606-21-3
116 suppliers
$45.95/1gm:

63365-23-1
28 suppliers
inquiry
