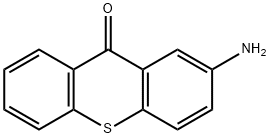
2-amino-9H-thioxanthen-9-one synthesis
- Product Name:2-amino-9H-thioxanthen-9-one
- CAS Number:33923-98-7
- Molecular formula:C13H9NOS
- Molecular Weight:227.29
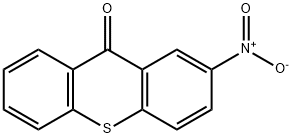
72534-70-4
1 suppliers
inquiry

33923-98-7
11 suppliers
$704.00/1g
Yield:-
Reaction Conditions:
with iron;ammonium chloride in ethanol;
Steps:
The syntheses of 2-methylaminothioxanthones 13 and 14 (eq 2 and Scheme 4) started with the corresponding known 2-nitro compounds 1810 and 1913, which were prepared by literature methods. The 2-nitro substituent was reduced to the amines 20 and 21 by iron,10 followed by acylation. The resultant carboxamides 22 and 23 were then alkylated to introduce the N-methyl substituents 24, 25. Subsequent base hydrolysis of the acetamides furnished the thioxanthone 2-methylamines 13 and 14.
References:
US2013/231484,2013,A1 Location in patent:Paragraph 0046
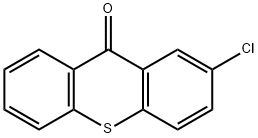
86-39-5
263 suppliers
$7.00/5g

33923-98-7
11 suppliers
$704.00/1g

20904-30-7
15 suppliers
inquiry

33923-98-7
11 suppliers
$704.00/1g
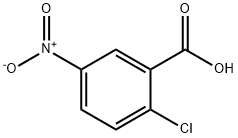
2516-96-3
460 suppliers
$6.00/25g

33923-98-7
11 suppliers
$704.00/1g
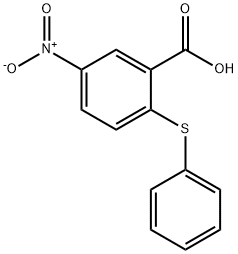
6345-67-1
13 suppliers
inquiry

33923-98-7
11 suppliers
$704.00/1g