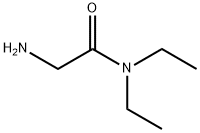
2-amino-N,N-diethylacetamide synthesis
- Product Name:2-amino-N,N-diethylacetamide
- CAS Number:34105-57-2
- Molecular formula:C6H14N2O
- Molecular Weight:130.19
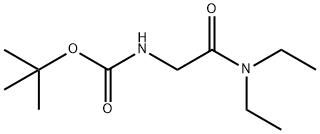
500871-60-3
18 suppliers
$45.00/100mg

34105-57-2
24 suppliers
$50.00/250mg
Yield:34105-57-2 1157 mg
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0 - 20;
Steps:
11. Preparation and characterization of TDGs Step 1:
General procedure: Step 1: A round bottom flask (100 mL) with magnetic stir bar was charged with Boc-glycine (20 mmol, 3.5 g). Then the flask was sealed and flushed with argon three times, 40 mL of anhydrous THF were syringed into the flask. Then the reaction mixture was cooled to -15 °C, and N-methylmorpholine (NMM) (2.48 mL, 22 mmol) was added, followed by dropwise addition of isobutyl chloroformate (2.8 mL, 22 mmol) to the reaction mixture. After stirring for 5 min, a solution of amine (20 mmol) in anhydrous THF and NMM (2.48 mL, 22 mmol) in anhydrous THF were added at the same time, and the reaction was stirred at -15 °C for 0.5 h. After that the reaction mixture was naturally warmed to room temperature and allowed to react overnight. After completion, the insoluble salt was removed by suction filtration. The filtrate was concentrated under reduced pressure and then dissolved with ethyl acetate. The ethyl acetate phase was washed successively with 1.0 M of sodium hydroxide solution, water, 1.0 M of hydrochloric acid solution and a small amount of saturated brine. After drying with anhydrous sodium sulfate, the solution was concentrated under reduced pressure to give Boc-glycinamide as a pale yellow oily liquid. Step 2: A round bottom flask (100 mL) with magnetic stir bar was charged withBoc-glycinamide (10 mmol), then 10 mL of dichloromethane was added. The reactionmixture was cooled to 0 °C. Trifluoroacetic acid (10 mL) was added dropwise to thereaction mixture. The reaction mixture was naturally warmed to room temperatureand allowed to react overnight. After completion, the reaction mixture wasconcentrated under reduced pressure and neutralized to pH = 12 using 1.0 M of soiumhydroxide solution. Then the solution was extracted with dichloromethane. Theresulting organic phase was dried over anhydrous sodium sulfate, and concentratedunder reduced pressure. The residue was isolated by column chromatography usingdichloromethane and methanol as eluents to give TDGs as products.
References:
Wen, Fei;Li, Zheng [Synthetic Communications,2020,vol. 50,# 22,p. 3462 - 3474] Location in patent:supporting information
![Benzyl N-[(diethylcarbaMoyl)Methyl]carbaMate](/CAS/20150408/GIF/79990-06-0.gif)
79990-06-0
8 suppliers
$131.00/1g

34105-57-2
24 suppliers
$50.00/250mg
![Benzeneacetic acid, 2-[(2,6-dichlorophenyl)amino]-, [[[[2-(diethylamino)-2-oxoethyl]amino]carbonyl]oxy]methyl ester](/CAS/20210305/GIF/934548-15-9.gif)
934548-15-9
0 suppliers
inquiry

34105-57-2
24 suppliers
$50.00/250mg

15307-86-5
366 suppliers
$46.00/25g
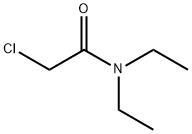
2315-36-8
255 suppliers
$17.00/5g

34105-57-2
24 suppliers
$50.00/250mg
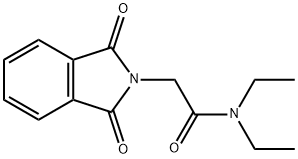
74169-74-7
1 suppliers
inquiry

34105-57-2
24 suppliers
$50.00/250mg