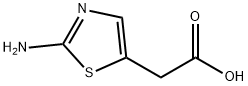
2-aminothiazol-5-acetic acid synthesis
- Product Name:2-aminothiazol-5-acetic acid
- CAS Number:52454-66-7
- Molecular formula:C5H6N2O2S
- Molecular Weight:158.18
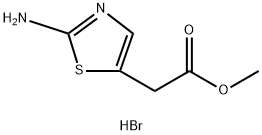
723278-34-0
29 suppliers
$112.00/250mg

52454-66-7
31 suppliers
$130.00/50mg
Yield: 100%
Reaction Conditions:
Stage #1:methyl 2-(2-aminothiazol-5-yl)acetate hydrobromide with sodium hydroxide;water in methanol at 20; for 3 h;
Stage #2: with hydrogenchloride in water; pH=3.5
Steps:
45.a
A solution of sodium hydroxide (30 g, 760 mmol) in water (60 ml) was added to a solution of methyl (2-AMINO-1, 3-thiazol-5-yl) acetate hydrobromide (see example 11) ) (64 g, 250 mmol) in methanol (600 ml) and the reaction was stirred at ambient temperature for 3 hours. The methanol was removed in vacuo and the residue acidified to pH 3.5 by addition of 1.0 N hydrochloric acid. The orange solid was collected by suction filtration, washed with water and dried in vacuo to yield (2-AMINO-1, 3-thiazol-5-yl) acetic acid (48.4 g, quantitative yield) as a pale orange solid: 'H-NMR (DMSO d6): 6.72 (br s, 2H), 6.69 (s, 1H), 3.54 (s, 2H) : MS (+ve ESI): 159 (M+H) +
References:
Location in patent:Page 123-124
62557-32-8
106 suppliers
$41.00/100mg

52454-66-7
31 suppliers
$130.00/50mg