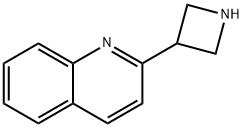
2-(azetidin-3-yl)quinoline dihydrochloride synthesis
- Product Name:2-(azetidin-3-yl)quinoline dihydrochloride
- CAS Number:1236861-65-6
- Molecular formula:C12H12N2
- Molecular Weight:184.24
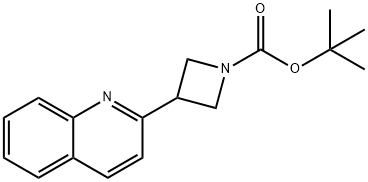
1236861-63-4
2 suppliers
inquiry

1236861-65-6
10 suppliers
inquiry
Yield:1236861-65-6 48%
Reaction Conditions:
Stage #1: tert-butyl 3-(quinolin-2-yl)azetidine-1-carboxylatewith hydrogenchloride in methanol at 20; for 18 h;
Stage #2: with sodium hydroxide in water;
Steps:
7.1.C
A solution of C36 (1.0 g, 3.5 mmol) in methanolic hydrochloric acid (1.25M, 50 mL, 62 mmol) was stirred at room temperature for 18 h. The reaction mixture was concentrated in vacuo, and extracted with dichloromethane after conversion of product to the free base with 6N aqueous sodium hydroxide solution. Removal of solvent in vacuo provided C37. Yield: 310 mg, 1.68 mmol, 48%. LCMS m/z 185.2 (M+1). 1H NMR (400 MHz, DMSO-d6) δ 3.80 (dd, J=8.0, 8.0 Hz, 2H), 3.91 (dd, J=7.4, 7.4 Hz, 2H), 4.16 (m, 1H), 7.54 (d, J=8.3 Hz, 1H), 7.56 (ddd, J=8.1, 6.9, 1.1 Hz, 1H), 7.74 (ddd, J=8.4, 6.9, 1.6 Hz, 1H), 7.96 (m, 2H), 8.32 (d, J=8.5 Hz, 1H).
References:
US2010/190771,2010,A1 Location in patent:Page/Page column 21
2005-43-8
205 suppliers
$6.00/250mg

1236861-65-6
10 suppliers
inquiry