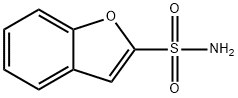
2-Benzofuransulfonamide(9CI) synthesis
- Product Name:2-Benzofuransulfonamide(9CI)
- CAS Number:124043-72-7
- Molecular formula:C8H7NO3S
- Molecular Weight:197.21
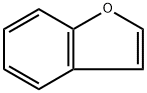
271-89-6
257 suppliers
$9.00/1g

124043-72-7
10 suppliers
inquiry
Yield:124043-72-7 76%
Reaction Conditions:
Stage #1: 1-benzofuranewith n-butyllithium in diethyl ether;hexane at -78; for 0.0833333 h;Inert atmosphere;
Stage #2: with N-sulfinyl-O-(tert-butyl)hydroxylamine in diethyl ether;hexane at -78; for 0.333333 h;Inert atmosphere;
Stage #3: with potassium hydroxide in diethyl ether;hexane;lithium hydroxide monohydrate at 60; for 6 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
General procedure for the synthesis of primary sulfonamides 14a-k:
General procedure: To a stirred solution of 13a-k (1.0 equiv.) in anhydrous Et2O (10.0 mL), was added 2.0 M n-BuLi in hexane (1.2 equiv) at -78 °C under nitrogen. The reaction mixture was stirred at -78 °C for 5 minutes, and then added t-BuONSO (1.2 equiv) in anhydrous Et2O (1.00 mL) drop wise. The resulting reaction mixture was stirred at -78 °C for 20 minutes added 2N KOH and stirred at 60 °C for 6 h. The reaction mixture was allowed to warm up to room temperature. The reaction mixture was extracted with EtOAc (3 x 10 mL). Combined organic layers were washed with brine (10.0 mL), dried over Na2SO4 and concentrated. The obtained crude residue was purified by column chromatography by eluting with 0 to 60% ethyl acetate/hexane to afford the pure primary sulfonamides 14a-k in 68-77% yield
References:
Tummanapalli, Satyanarayana;Bodige, Srinu;Gulipalli, Kali Charan;Endoori, Srinivas;Medaboina, Srinivas;Mallidi, Kalyani [Tetrahedron Letters,2022,vol. 97,art. no. 153781] Location in patent:supporting information
7664-41-7
502 suppliers
$15.00/100ml

124043-72-7
10 suppliers
inquiry

17070-58-5
49 suppliers
$94.25/500mg

124043-72-7
10 suppliers
inquiry