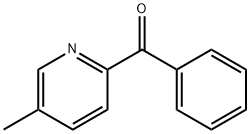
2-Benzoyl-5-methylpyridine synthesis
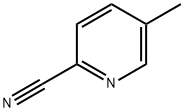
- Product Name:2-Benzoyl-5-methylpyridine
- CAS Number:127581-43-5
- Molecular formula:C13H11NO
- Molecular Weight:197.23

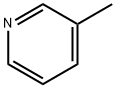
63065-67-8
5 suppliers
inquiry

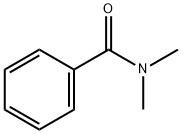
127581-43-5
8 suppliers
inquiry
Yield:127581-43-5 82%
Reaction Conditions:
with copper(l) iodide;oxygen;acetic acid in dimethyl sulfoxide at 100 - 130; under 760.051 Torr; for 24 h;
Steps:
1. General procedure A
General procedure: Two 10 mL vials were subsequently charged with CuI (0.0095 g, 0.05 mmol, 10 mol %) or FeCl2·4H2O (0.0094 g, 0.05 mmol, 10 mol %), substrate (0.5 mmol), DMSO (1 mL) and acetic acid (0.030 g, 0.5 mmol). The vials were flushed with O2 for 1 min, capped with an aluminum crimp cap/septum and finally stirred at 100 °C for 24 h in an oil bath with a balloon filled with O2 through the septum. After cooling to room temperature, the content of the vials was transferred into a separation funnel and the vials were rinsed with dichloromethane (20 mL). Sodium bicarbonate solution (10 mL, sat.) was added and the organic phase was separated. The aqueous phase was extracted twice with dichloromethane (10 mL). The combined organic fractions were washed with brine (20 mL), dried over MgSO4 and filtered over a pad of Celite. The solvent was removed under reduced pressure and the resulting residue was purified by column chromatography with an automated chromatography system using a silica flash cartridge (40 g) applying a heptane/ethyl acetate gradient (from 100% heptane to 100% ethyl acetate in 25 minutes, 25 mL/min).
References:
Sterckx, Hans;De Houwer, Johan;Mensch, Carl;Herrebout, Wouter;Tehrani, Kourosch Abbaspour;Maes, Bert U.W. [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 144 - 153] Location in patent:supporting information
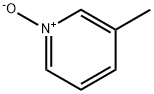
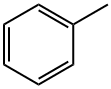
1003-73-2
280 suppliers
$9.00/5g
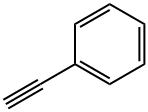
536-74-3
295 suppliers
$10.00/1g

127581-43-5
8 suppliers
inquiry

27693-44-3
8 suppliers
inquiry