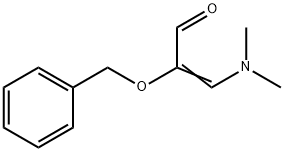
2-Benzyl-3-(dimethylamino)acrolein synthesis
- Product Name:2-Benzyl-3-(dimethylamino)acrolein
- CAS Number:143462-35-5
- Molecular formula:C12H15NO2
- Molecular Weight:205.25

42783-78-8
67 suppliers
$58.00/10g

143462-35-5
17 suppliers
inquiry
Yield:143462-35-5 53%
Reaction Conditions:
Stage #1: benzyloxyacetaldehyde diethyl acetalwith phosphorus pentachloride at 0 - 75; for 1.75 h;
Stage #2: with N,N-dimethyl-formamide at 20; for 72 h;
Stage #3: with sodium hydroxide in water at 0; pH=8;
Steps:
1.1.d d)
[(2,2-Diethoxyethoxy)methyl]benzene (8.30 g, 37.0 mmol) stirred under ice cooling was added with phosphorus pentachloride (8.09 g, 38.8 mmol) over 15 minutes. The mixture was stirred at the same temperature for 15 minutes, and then heated and stirred on an oil bath at 75° C. for 75 minutes. The reaction mixture was cooled by stirring at room temperature for 20 minutes, and then added with anhydrous N,N-dimethylformamide (8.6 mL, 111 mmol) at the same temperature, and the mixture was stirred at room temperature for 3 days. The reaction mixture was added with 8 M aqueous sodium hydroxide on an ice bath until pH became 8 or higher, and then the mixture was diluted with water, and extracted with ether. The organic layer was washed successively with water and saturated brine, dried over anhydrous sodium sulfate, and then concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane:acetone=4:1→2:1) to obtain 2-(benzyloxy)-3-(dimethylamino)acrylaldehyde (4.05 g, 53%) as a brown oil.1H-NMR (CDCl3) δ: 3.04 (6H, s), 4.96 (2H, s), 6.17 (1H, s), 7.28-7.43 (5H, m), 8.64 (1H, s).
References:
US2009/82352,2009,A1 Location in patent:Page/Page column 22