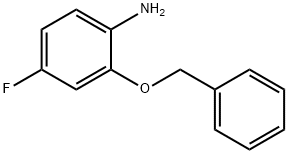
2-(benzyloxy)-4-fluoroaniline synthesis
- Product Name:2-(benzyloxy)-4-fluoroaniline
- CAS Number:159471-73-5
- Molecular formula:C13H12FNO
- Molecular Weight:217.24
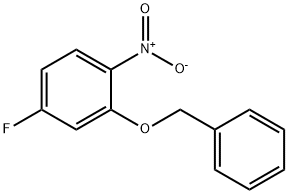
129464-01-3
28 suppliers
$65.00/500mg

159471-73-5
23 suppliers
$110.00/100mg
Yield:159471-73-5 1.26 g
Reaction Conditions:
with iron;ammonium chloride in ethanol at 100; for 1 h;
Steps:
16-1.2 2-(Benzyloxy)-4-fluoroaniline
To an ethanol (25 mL)-water (25 mL) mixture containing 2-(benzyloxy)-4-fluoro-1-nitrobenzene obtained were added iron (2.79 g, 50.0 mmol) and ammonium chloride (535 mg, 10.0 mmol) at room temperature, and the mixture was stirred at 100°C for 1 hr.
After cooling to room temperature, the reaction mixture was filtered through Celite (registered trademark) and the filtrate was concentrated under reduced pressure.
Saturated aqueous sodium hydrogen carbonate solution was added to the resulting residue, and the mixture was extracted three times with ethyl acetate.
The organic layer was washed with brine and dried over anhydrous magnesium sulfate and then the desiccant was filtered off, followed by concentration under reduced pressure.
The resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate = 9:1) to afford the title compound as a brownish red oil (1.26 g).
1H NMR (300 MHz, CDCl3) δ ppm 5.05 (s, 2 H), 6.47 - 6.57 (m, 1 H), 6.58 - 6.72 (m, 2 H), 7.29 - 7.48 (m, 5 H).
MS ESI/APCI Dual posi: 218[M+H]+.
References:
EP2687507,2014,A1 Location in patent:Paragraph 0315
446-36-6
255 suppliers
$9.00/10g

100-39-0
425 suppliers
$10.00/10g

159471-73-5
23 suppliers
$110.00/100mg