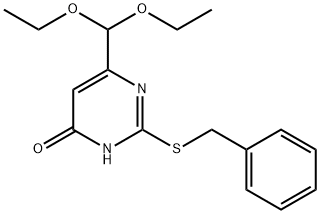
2-Benzylsulfanyl-6-diethoxymethyl-pyrimidin-4-ol synthesis
- Product Name:2-Benzylsulfanyl-6-diethoxymethyl-pyrimidin-4-ol
- CAS Number:525559-02-8
- Molecular formula:C16H20N2O3S
- Molecular Weight:320.41

100-39-0
436 suppliers
$10.00/10g
10495-09-7
49 suppliers
$65.00/50mg

17356-08-0
3 suppliers
inquiry

525559-02-8
9 suppliers
inquiry
Yield:525559-02-8 76%
Reaction Conditions:
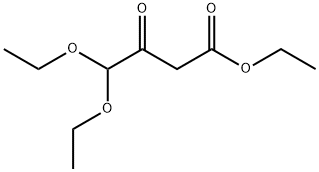
Stage #1: ethyl 4,4-diethoxyacetoacetate;thioureawith sodium methylate in methanol;ethanol; for 16 h;Reflux;
Stage #2: benzyl bromide in methanol;ethanol;water;
Steps:
83
Intermediate 83: 6-[bis(ethyloxy)methyl]-2-[(phenylmethyl)thio]-4(1H)-pyrimidinoneA mixture of ethyl 4,4-bis(ethyloxy)-3-oxobutanoate (34.6g, 159mmol) and thiourea (13.27g, 174mmol) in ethanol (15OmL) was treated with sodium methoxide (25% in methanol) (36mL, 159mmol) and the mixture was heated to reflux for 4 hours and then allowed to stand for 12 hours. Water (15OmL) was added and the stirred mixture was treated with benzyl bromide (18.9mL, 159mmol). After 5 minutes the stirring was ceased and a white precipitate formed. After 1 hour the mixture was diluted with water (50OmL) and the precipitated solid was filtered off, washed with water and thoroughly dried to afford the title compound (38.7g, 121 mmol, 76% yield). LCMS (Method A): Rt 1.03 minutes; m/z 321 (MH+).
References:
WO2010/106016,2010,A1 Location in patent:Page/Page column 126