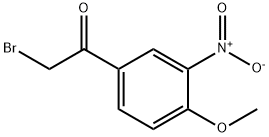
2-Bromo-1-(4-methoxy-3-nitrophenyl)-1-ethanone synthesis
- Product Name:2-Bromo-1-(4-methoxy-3-nitrophenyl)-1-ethanone
- CAS Number:65447-49-6
- Molecular formula:C9H8BrNO4
- Molecular Weight:274.07
6277-38-9
94 suppliers
$18.00/1g

65447-49-6
17 suppliers
$170.00/100mg
Yield:65447-49-6 67%
Reaction Conditions:
with bromine in chloroform at 20; for 0.5 h;
Steps:
3.A.i.1 Example 3: synthesis of amine functionalized 2-aryl, 3-aroyl substituted indoles and corresponding phosphoramidate prodrugs; A) 2-aryl,3-aroyl indole phosphoramidate prodrug ; i) 4'-methoxy-3'-nitro-2-bromoacetophenone (22)
i) 4'-methoxy-3'-nitro-2-bromoacetophenone (22) : to a well-stirred solution of 3-nitro-4-methoxyacetophenone 21 (5.334 g, 27.33 mmol) in chloroform (25 ml) at room temperature, was added a solution of bromine (1.40 ml, 27.33 mmol, 1.0 equivalent) in chloroform (5 ml), slowly dropwise. the reaction mixture was stirred for 30 minutes, periodically checking by tlc. then water (25 ml) was added, organic layer was separated and the aqueous layer was extracted with chc13 (3 x 15 ml). the combined organic phases were dried over anhydrous na2s04, filtered and rotovaped to afford a white- colored solid. this crude product was then recrystallized from hot ethanol (40 ml) to yield the desired bromoacetophenone derivative 22 as cream-colored, needle-shaped crystals (5.038 g, 18.38 mmol, 67%), rf= 0.286 (etoac/hexanes: 40/60). During this procedure a small amount of 4'-methoxy-3'-nitro-2,2- dibromoacetophenone is also formed, Rf= 0.404 (etoac/hexanes: 40/60). when the reaction was continued further beyond 30 minutes, it was observed that the monobromo derivative got converted to the dibromo derivative. 1H NMR (300 MHz, CDC13) : δ 8.48 (1H, d, J= 2.3 Hz), 8.21 (1H, dd, J= 2.2 Hz, J= 8.8 Hz), 7.20 (1H, d, J= 8.9 Hz), 4.40 (2H, s), 4.07 (3H, s). 13C NMR (75 MHZ, CDC13) : δ 188.45, 162.25, 156.68, 134.82, 126.76, 126.13, 113.55, 57.00, 29.89. GC-MS: retention time = 14.36 minutes (120oc at the rate of 10oc/minute).
References:
WO2004/99139,2004,A1 Location in patent:Page 34