
2-BROMO-1-(5-BROMO-2-METHOXYPHENYL)ETHANONE synthesis
- Product Name:2-BROMO-1-(5-BROMO-2-METHOXYPHENYL)ETHANONE
- CAS Number:67639-58-1
- Molecular formula:C9H8Br2O2
- Molecular Weight:307.97
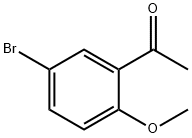
16740-73-1
62 suppliers
$15.00/1g

67639-58-1
29 suppliers
$99.70/1g
Yield:67639-58-1 78%
Reaction Conditions:
with N-Bromosuccinimide;toluene-4-sulfonic acid in neat (no solvent) at 60; for 0.25 h;regioselective reaction;
Steps:
General procedure for the synthesis of phenacyl bromides (3a-i)
General procedure: The round-bottom flask containing acetophenone derivative 2a-i (4 mmol) and PTSA (0.076 g, 0.4 mmol) was heated to 60 oC to turn the reaction mixture into a paste and then NBS (0.854 g, 4.8 mmol) was added slowly. After 15 min., the reaction mixture was cooled to room temperature and water (20 ml) was added. The crude product was extracted with dichloromethane (2 x 20 mL), dried over anhydrous Na2SO4 and puried by crystallization from hexane-dichloromethane to give pure product.
References:
Phan, Phuong-Thuy T.;Nguyen, Hong-Nhung T.;Kim, Son N.;Pham, Tuan-Anh N. [Synthetic Communications,2021,vol. 51,# 5,p. 786 - 796] Location in patent:supporting information
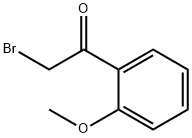
31949-21-0
145 suppliers
$34.00/5g

67639-58-1
29 suppliers
$99.70/1g
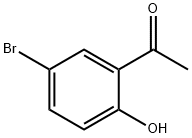
1450-75-5
312 suppliers
$10.00/1g

67639-58-1
29 suppliers
$99.70/1g
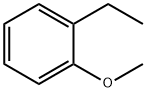
14804-32-1
56 suppliers
$45.00/50mg

67639-58-1
29 suppliers
$99.70/1g