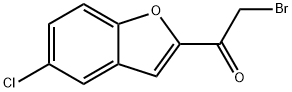
2-BROMO-1-(5-CHLORO-1-BENZOFURAN-2-YL)ETHANONE synthesis
- Product Name:2-BROMO-1-(5-CHLORO-1-BENZOFURAN-2-YL)ETHANONE
- CAS Number:7039-74-9
- Molecular formula:C10H6BrClO2
- Molecular Weight:273.51
![1-(5-CHLOROBENZO[B]FURAN-2-YL)ETHAN-1-ONE](/CAS/GIF/1646-32-8.gif)
1646-32-8
29 suppliers
$58.00/250mg

7039-74-9
19 suppliers
inquiry
Yield:7039-74-9 50%
Reaction Conditions:
with copper(ll) bromide in ethyl acetate at 20 - 80; for 4 h;Inert atmosphere;
Steps:
55 2-bromo-]-(5-chlorobenzofuran-2-yl) ethan-] -one:
To a stirred solution of 1-(5- chlorobenzofuran-2-yl) ethan-1-one (4.2 g, 22 mmol) in EtOAc: CHC13 (3: 1, 84 mL) at room temperature under an argon atmosphere was added copper bromide (10.63 g, 48 mmol). The reaction mixture was stirred for 4 h at 80 °C. After consumption of starting material (monitored by TLC), the reaction mixture was filtered and the filtrate was concentrated in vacuo. The crude material was purified by column chromatography using 2-3% EtOAc:Hexane to afford 2-bromo-1-(5-chlorobenzofuran-2-yl) ethan-1-one (3 g, 50%) as a pale yellow solid used in the next step without further purification.[0395] ‘H NMR (DMSO-d6, 400 MHz): 8.00 (s, 1H), 7.97 (s, 1H), 7.79 (d, 1H), 7.58 (dd, 1H), 4.81 (s, 2H); LCMS: 96.2%; 273 (M+2); (column; X-select CSH C-18 (50 x 3.0 mm, 3.5 pm); RT 4.34 mm; mobile phase: 5 mlvi Aq NH4OAc: ACN; T/B%: 0.0 1/10, 0.5/10, 4/90, 9/90; flow rate: 0.8 mL/min) (Gradient); TLC: 5% EtOAc/ Hexane (R1 0.3).
References:
WO2016/201168,2016,A1 Location in patent:Paragraph 0394; 0395

635-93-8
348 suppliers
$6.00/5g

7039-74-9
19 suppliers
inquiry