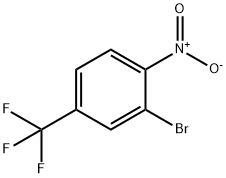
2-Bromo-1-nitro-4-(trifluoromethyl)benzene synthesis
- Product Name:2-Bromo-1-nitro-4-(trifluoromethyl)benzene
- CAS Number:132839-58-8
- Molecular formula:C7H3BrF3NO2
- Molecular Weight:270
57946-63-1
236 suppliers
$10.00/5g

132839-58-8
73 suppliers
$13.00/250mg
Yield: 60%
Reaction Conditions:
with sodium perborate tetrahydrate;acetic acid at 55; for 7 h;
Steps:
Preparation of 2-bromo-1-nitro-4-(trifluoromethyl)benzene
This compound was prepared by application of the procedure for oxidation of4-(trifluoromethyl)aniline.[7] A 300 mL three-necked round-bottom flask equipped with a refluxcondenser and a dropping funnel was charged with sodium perborate tetrahydrate (40.69 g, 0.264mol) and glacial acetic acid (50 mL). The dropping funnel was charged with2-bromo-4-(trifluoromethyl)aniline (6.31 g, 26.8 mmol) and glacial acetic acid (50 mL). Thesuspension in the flask was heated to 55°C and the content of the funnel was added dropwise to it.After 3 h, an additional sodium perborate tetrahydrate (20.00 g, 0.130 mol) was added to the reactionmixture and stirred at 55°C for 4 h. The resulting yellow suspension was cooled to room temperatureand filtered through a Celite pad. To the filtrate was added diethyl ether (200 mL) and water (100mL). The organic phase was separated from the mixture and aqueous phase was extracted withdiethyl ether (150 mL x 2). Combined organic phase was neutralized by aqueous sodium hydroxidesolution (3 M) and separated. The separated organic phase was dried over sodium sulfate andconcentrated to afford brown liquid. This crude product was purified by flash columnchromatography (12% EtOAc/hexane) to give the title compound as a yellow liquid (4.22 g, 60 %).
References:
Takahashi, Hirotsugu;Watanabe, Takahito;Tobita, Hiromi [Chemistry Letters,2018,vol. 47,# 3,p. 296 - 299] Location in patent:supporting information

402-14-2
101 suppliers
$20.00/250mg

132839-58-8
73 suppliers
$13.00/250mg