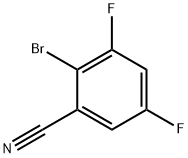
2-broMo-3,5-difluorobenzonitrile synthesis
- Product Name:2-broMo-3,5-difluorobenzonitrile
- CAS Number:425379-37-9
- Molecular formula:C7H2BrF2N
- Molecular Weight:217.9983
64248-63-1
298 suppliers
$6.00/5g

425379-37-9
13 suppliers
inquiry
Yield:425379-37-9 99%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;sulfuric acid in dichloromethane;acetonitrile at 50; for 4 h;
Steps:
8.a 2',4,6-Trifluoro-5'- [8-fluoro-7-(1-hydroxy-1-methylethyl) IMIDA ZO [LZ2-CPYRIMIDIN-3-VLJ-L L'-BIPHENYL-2-CARBOLLITRILE a) 2-Bromo-3, 5-difluorobenzonitrile
Dichloromethane (150 ml) was added to a slurry of 3,5- difluorobenzonitrile (10.0 g, 71.9 mmol) and 1, 3-dibromo-5,5- dimethylhydantoin (20.6 g, 71.9 mmol) in acetonitrile (11.8 g, 287 mmol). The mixture was cooled to 0C then concentrated sulfuric acid (28.2 g, 287 mmol) was added dropwise to the solution over 30 min. On completion of the addition the mixture was allowed to warm to room temperature then heated at 50 OC for 4 h. 1 N Sodium sulfite solution (200 ml) and dichloromethane (300 ml) were added and the layers separated. The organic phase was washed with 1 N sodium sulfite solution (200 ml) and water (200 ml), dried over anhydrous sodium sulfate, filtered and evaporated to give 15. 6 g (99%) of the title compound as an off white solid: ON (400 MHz, CDCL3) 7.22-7. 14 (1 H, m), 7.29 (1 H, m).
References:
WO2004/65388,2004,A1 Location in patent:Page 45